Advertisements
Advertisements
Question
Hydrochloric acid reacts with a metal X to form a gas Y which burns with a 'pop' sound. Sodium hydroxide solution also reacts with the same metal X (on heating) to form the same gas Y.
Write the chemical equation of the reaction of metal X with (i) hydrochloric acid, and (ii) sodium hydroxide solution.
Solution
The chemical equation is as follows.
(i) Zn + 2HCl→ZnCl2 + H2 ↑
(ii) 2NaOH + Zn→Na2ZnO2 + H2
APPEARS IN
RELATED QUESTIONS
Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. In which test tube will the fizzing occur more vigorously, and why?
With the help of labelled diagrams, describe an activity to show that acids produce ions only in aqueous solutions.
What ions are present in the solutions of following substances? (write the symbols only)
Hydrochloric acid
With which substance should chlorine be treated to get bleaching powder?
What does a soda-acid type fire extinguisher contain? How does it work? Explain the working of a soda-acid fire extinguisher with the help of a labelled diagram.
State two uses each of the following compounds:
Hydrochloric acid
Which of the following gives the correct increasing order of acidic strength?
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
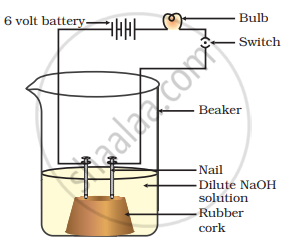
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
For each of the salt: A, B, C and D, suggest a suitable method of its preparation.
C is a soluble salt of copper.
