Advertisements
Advertisements
प्रश्न
Hydrochloric acid reacts with a metal X to form a gas Y which burns with a 'pop' sound. Sodium hydroxide solution also reacts with the same metal X (on heating) to form the same gas Y.
Write the chemical equation of the reaction of metal X with (i) hydrochloric acid, and (ii) sodium hydroxide solution.
उत्तर
The chemical equation is as follows.
(i) Zn + 2HCl→ZnCl2 + H2 ↑
(ii) 2NaOH + Zn→Na2ZnO2 + H2
APPEARS IN
संबंधित प्रश्न
When acids react with metal, which gas is liberated?
Complete and balance the following chemical equations:
Zn (s) + HCI (aq) →
What happens when an acid reacts with a metal?
A substance X which is used as an antacid reacts with dilute hydrochloric acid to produce a gas Y which is used in one type of fire-extinguisher. Name the substance X and gas Y. Write a balanced equation for the chemical reaction which takes place.
Phenolphthalein is a synthetic type of indicator.
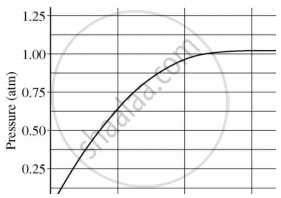
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
- The temperature of the solution increases
- The temperature of the solution decreases
- The temperature of the solution remains the same
- Salt formation takes place
______ change the colour of the indicators.
What property do acids and bases have in common? Explain it with an example.
Consider the following salt:
YCl
What would be the pH of the solution if, in YCl, Y is sodium? Give a reason for your answer.
