Advertisements
Advertisements
प्रश्न
Answer the following question.
Why is the wave theory of electromagnetic radiation not able to explain the photoelectric effect? How does a photon picture resolve this problem?
उत्तर
There are three main drawbacks:
- Intensity: If we consider light as a wave then as the intensity of the light is increased the amplitude of the oscillation of the electron will increase. Thus, as the intensity of the incident light is increased the maximum kinetic energy of the emitted electron will also increase. But it was observed that the kinetic energy of the emitted electrons does not depend on the intensity whereas the magnitude of the photoelectric current increases with the frequency.
- Frequency: If we consider the light as a wave then the photoelectric emission should happen on any frequency, but it was observed that the electrons are emitted after a particular frequency. If the frequency of the incident light is lesser than this frequency there is no photoelectric emission observed.
- Time Delay: According to the wave theory the energy is uniformly distributed over the wavefront. As the light falls on the metallic surface, it will take some time for the electron to gain sufficient energy to get emitted. But experimentally it was observed that the electrons are emitted instantaneously as the light falls on the metallic surface.
How the photon theory can explain the photoelectric effect:
- According to photon theory increasing the intensity means increasing the number of photons that do not change the maximum kinetic energy but changes the number of ejected electrons.
- The energy of a photon is given as E = hf that explains the dependence of the energy on the frequency, after a particular frequency of a photon that is threshold frequency there is photoelectric emission.
- As soon as a photon falls on the metallic surface it is absorbed, hence the electron is ejected instantaneously. Hence, all these are in accordance with the experimental observations.
संबंधित प्रश्न
An electron gun with its collector at a potential of 100 V fires out electrons in a spherical bulb containing hydrogen gas at low pressure (∼10−2 mm of Hg). A magnetic field of 2.83 × 10−4 T curves the path of the electrons in a circular orbit of radius 12.0 cm. (The path can be viewed because the gas ions in the path focus the beam by attracting electrons, and emitting light by electron capture; this method is known as the ‘fine beam tube’ method. Determine e/m from the data.
Define the term "cut off frequency" in photoelectric emission. The threshod frequency of a metal is f. When the light of frequency 2f is incident on the metal plate, the maximum velocity of photo-electrons is v1. When the frequency of the incident radiation is increased to 5f, the maximum velocity of phto-electrons is v2. Find the ratio v1 : v2.
A horizontal cesium plate (φ = 1.9 eV) is moved vertically downward at a constant speed v in a room full of radiation of wavelength 250 nm and above. What should be the minimum value of v so that the vertically-upward component of velocity is non-positive for each photoelectron?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
In the arrangement shown in the figure, y = 1.0 mm, d = 0.24 mm and D = 1.2 m. The work function of the material of the emitter is 2.2 eV. Find the stopping potential V needed to stop the photocurrent.
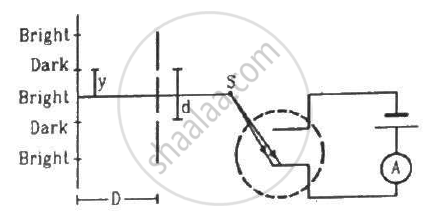
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Plot a graph to show the variation of stopping potential with frequency of incident radiation in relation to photoelectric effect.
In the experimental set up for studying photoelectric effect, if keeping the frequency of the incident radiation and the accelerating potential fixed, the intensity of light is varied, then ______.
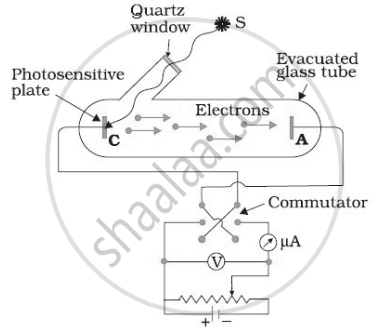
For a given frequency of light and a positive plate potential in the set up below, If the intensity of light is increased then ______.
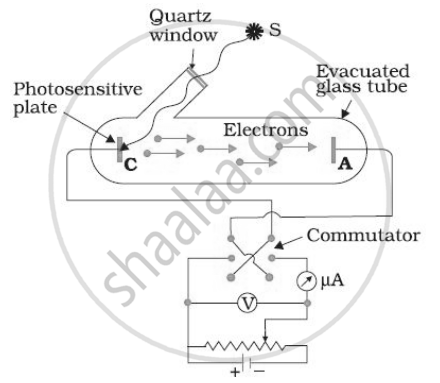
In various experiments on photo electricity, the stopping potential for a given frequency of the incident radiation is ______.
When a beam of 10.6 eV photons of intensity 2.0 W/m2 falls on a platinum surface of area 1.0 × 10-4 m2, only 53% of the incident photons eject photoelectrons. The number of photoelectrons emitted per second is ______.
The electromagnetic theory of light failed to explain ______.
