Advertisements
Advertisements
प्रश्न
Answer the following question.
Why is the wave theory of electromagnetic radiation not able to explain the photoelectric effect? How does a photon picture resolve this problem?
उत्तर
There are three main drawbacks:
- Intensity: If we consider light as a wave then as the intensity of the light is increased the amplitude of the oscillation of the electron will increase. Thus, as the intensity of the incident light is increased the maximum kinetic energy of the emitted electron will also increase. But it was observed that the kinetic energy of the emitted electrons does not depend on the intensity whereas the magnitude of the photoelectric current increases with the frequency.
- Frequency: If we consider the light as a wave then the photoelectric emission should happen on any frequency, but it was observed that the electrons are emitted after a particular frequency. If the frequency of the incident light is lesser than this frequency there is no photoelectric emission observed.
- Time Delay: According to the wave theory the energy is uniformly distributed over the wavefront. As the light falls on the metallic surface, it will take some time for the electron to gain sufficient energy to get emitted. But experimentally it was observed that the electrons are emitted instantaneously as the light falls on the metallic surface.
How the photon theory can explain the photoelectric effect:
- According to photon theory increasing the intensity means increasing the number of photons that do not change the maximum kinetic energy but changes the number of ejected electrons.
- The energy of a photon is given as E = hf that explains the dependence of the energy on the frequency, after a particular frequency of a photon that is threshold frequency there is photoelectric emission.
- As soon as a photon falls on the metallic surface it is absorbed, hence the electron is ejected instantaneously. Hence, all these are in accordance with the experimental observations.
संबंधित प्रश्न
Is the formula you employ in (a) valid for calculating radius of the path of a 20 MeV electron beam? If not, in what way is it modified?
An electron gun with its collector at a potential of 100 V fires out electrons in a spherical bulb containing hydrogen gas at low pressure (∼10−2 mm of Hg). A magnetic field of 2.83 × 10−4 T curves the path of the electrons in a circular orbit of radius 12.0 cm. (The path can be viewed because the gas ions in the path focus the beam by attracting electrons, and emitting light by electron capture; this method is known as the ‘fine beam tube’ method. Determine e/m from the data.
If light of wavelength 412.5 nm is incident on each of the metals given below, which ones will show photoelectric emission and why?
| Metal | Work Function (eV) |
| Na | 1.92 |
| K | 2.15 |
| Ca | 3.20 |
| Mo | 4.17 |
A photographic film is coated with a silver bromide layer. When light falls on this film, silver bromide molecules dissociate and the film records the light there. A minimum of 0.6 eV is needed to dissociate a silver bromide molecule. Find the maximum wavelength of light that can be recorded by the film.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
In an experiment on photoelectric effect, light of wavelength 400 nm is incident on a cesium plate at the rate of 5.0 W. The potential of the collector plate is made sufficiently positive with respect to the emitter, so that the current reaches its saturation value. Assuming that on average, one out of every 106 photons is able to eject a photoelectron, find the photocurrent in the circuit.
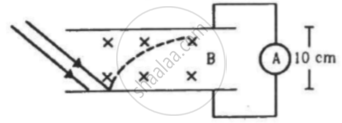
In an experiment on photoelectric effect, the emitter and the collector plates are placed at a separation of 10 cm and are connected through an ammeter without any cell. A magnetic field B exists parallel to the plates. The work function of the emitter is 2.39 eV and the light incident on it has wavelengths between 400 nm and 600 nm. Find the minimum value of B for which the current registered by the ammeter is zero. Neglect any effect of space charge.
A silver ball of radius 4.8 cm is suspended by a thread in a vacuum chamber. Ultraviolet light of wavelength 200 nm is incident on the ball for some time during which light energy of 1.0 × 10−7 J falls on the surface. Assuming that on average, one photon out of every ten thousand is able to eject a photoelectron, find the electric potential at the surface of the ball, assuming zero potential at infinity. What is the potential at the centre of the ball?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The stopping potential in an experiment on photoelectric effect is 1.5V. What is the maximum kinetic energy of the photoelectrons emitted? Calculate in Joules.
In the case of a photo electric effect experiment, explain the following facts, giving reasons.
The wave theory of light could not explain the existence of the threshold frequency.
An increase in the intensity of the radiation causing photo-electric emission from a surface does not affect the maximum K.E. of the photoelectrons. Explain.
