Advertisements
Advertisements
प्रश्न
Arrange the following in the increasing order of their pKb values:
C6H5NH2, C2H5NH2, C6H5NHCH3
उत्तर
Order of pKb values-
C2H5NH2 < C6H5NHCH3 < C6H5NH2
This is simply because +I effect of C2H5 group increases the basicity of amine while -I effect of C6H5 group decreases the basicity of Amine.
APPEARS IN
संबंधित प्रश्न
Write a short note on Hoffmann bromamide degradation.
Illustrate the following reaction giving suitable example in each case:Gabriel phthalimide synthesis
Give the structures of A, B and C in the following reactions:
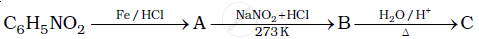
Give the structures of A, B and C in the following reaction:
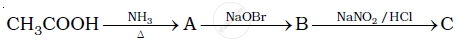
Write the reactions of aromatic with nitrous acid.
Which of the following amines forms a clear solution when treated with benzene sulphonyl chloride and excess of potassium hydroxide?
Identify A and B in the following reaction.
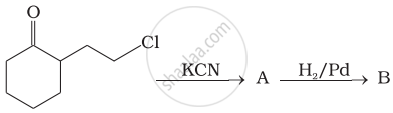
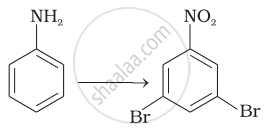
How will you carry out the following conversions?
Assertion: Aromatic 1° amines can be prepared by Gabriel Phthalimide Synthesis.
Reason: Aryl halides undergo nucleophilic substitution with anion formed by phthalimide.
The amine 'A' when treated with nitrous acid gives yellow oily substance. The amine A is ______.
