Advertisements
Advertisements
प्रश्न
Arrange the following in the increasing order of their pKb values:
C6H5NH2, C2H5NH2, C6H5NHCH3
उत्तर
Order of pKb values-
C2H5NH2 < C6H5NHCH3 < C6H5NH2
This is simply because +I effect of C2H5 group increases the basicity of amine while -I effect of C6H5 group decreases the basicity of Amine.
APPEARS IN
संबंधित प्रश्न
Give the structures of A, B and C in the following reaction:
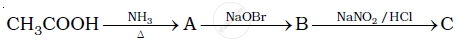
Write the reactions of aromatic with nitrous acid.
Explain Hoffmann’s exhaustive alkylation with suitable reactions.
Write reactions for the preparation of ethanamine using Gabriel phthalimide synthesis.
Which nitrogen containing compound amongst the following would undergo Mendius reduction to furnish primary amine \[\ce{(R - NH2)}\]?
Quaternary ammonium salt is formed:
Which of the following methods of preparation of amines will give same number of carbon atoms in the chain of amines as in the reactant?
Which of the following CANNOT be prepared by ammonolysis of alkyl halide?
Write the name of the product formed by the action of LiAlH4/ether on acetamide.
Write a short note on Ammonolysis.
