Advertisements
Advertisements
प्रश्न
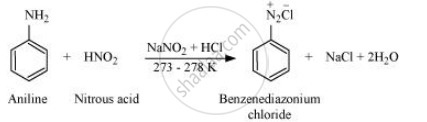
Write the reactions of aromatic with nitrous acid.
उत्तर
Aromatic amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at 273 − 278 K to form stable aromatic diazonium salts i.e., NaCl and H2O.
APPEARS IN
संबंधित प्रश्न
Accomplish the following conversions Nitrobenzene to benzoic acid
Give plausible explanation for each of the following :
Why are amines less acidic than alcohols of comparable molecular masses?
Arrange the following in the increasing order of their pKb values:
C6H5NH2, C2H5NH2, C6H5NHCH3
Account for the following:
Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
Choose the most correct option.
Which of the following compounds will dissolve in aqueous NaOH after undergoing reaction with Hinsberg reagent?
Answer the following
Explain Gabriel phthalimide synthesis.
Write reactions for the preparation of ethanamine using Gabriel phthalimide synthesis.
The end product C of the following reaction is
\[\ce{C2H5NH2 ->[HNO2] A ->[PCl5] B ->[NH3][Alcohol] C}\]
\[\ce{CH3-CN ->[Na/C2H5OH]}\]
The product formed is ____________.
Which of the following amines cannot be prepared by Gabriel phthalimide synthesis?
The best reagent for converting, 2-phenylpropanamide into 1- phenylethanamine is ______.
Among the following amines, the strongest Brönsted base is:
Which of the following amines can be prepared by Gabriel synthesis.
(i) Isobutyl amine
(ii) 2-Phenylethylamine
(iii) N-methylbenzylamine
(iv) Aniline
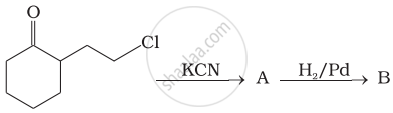
Identify A and B in the following reaction.
Assertion: Only a small amount of \[\ce{HCl}\] is required in the reduction of nitro compounds with iron scrap and \[\ce{HCl}\] in the presence of steam.
Reason: \[\ce{FeCl2}\] formed gets hydrolysed to release \[\ce{HCl}\] during the reaction.
A primary amine is formed by an amide on treatment with bromine and alkali. The primary amine has
A compound 'A' on reduction with iron scrap and hydrochloric acid gives compound 'B' with molecular formula C6H7N. Compound 'B' on reaction with CHCl3 and alcoholic KOH produces an obnoxious smell of carbylamine due to the formation of 'C'. Identify 'A', 'B' and 'C' and write the chemical reactions involved.
What is the IUPAC name of \[\ce{(CH3)2 - N - CH3}\]?
Write a short note on the following:
Ammonolysis
Write the name of reduction product formed when ethyl cyanide is treated with sodium and alcohol.
