Advertisements
Advertisements
प्रश्न
Give plausible explanation for each of the following :
Why are amines less acidic than alcohols of comparable molecular masses?
उत्तर १
Amines undergo protonation to give amide ion.
`R- NH_2 -> R - bar(N) H + H^(+)`
Amide ion
Similarly, alcohol loses a proton to give alkoxide ion.
`R - OH -> R - bar(O) + H^(+)`
Alcohol Alkoxide ion
In an amide ion, the negative charge is on the N-atom whereas in alkoxide ion, the negative charge is on the O-atom. Since O is more electronegative than N, O can accommodate the negative charge more easily than N. As a result, the amide ion is less stable than the alkoxide ion. Hence, amines are less acidic than alcohols of comparable molecular masses
उत्तर २
Loss of proton from an amine gives an amide ion while loss of a proton from alcohol give an alkoxide ion.
R—NH2—>R—NH– +H+
R—O —H—>R— O– +H+ .
Since O is more electronegative than N, so it wijl attract positive species more strongly in comparison to N. Thus, RO~ is more stable than RNH®. Thus, alcohols are more acidic than amines. Conversely, amines are less acidic than alcohols.
APPEARS IN
संबंधित प्रश्न
How are propan-1-amine and propan-2-amine prepared from oxime?
Identify the compounds 'A' and 'B' in the following equation:

Write short notes on the following Hofmann’s bromamide reaction
The following amines is the product of Gabriel phthalimide synthesis.
Identify the product 'A' in the following reaction.
\[\ce{Aniline ->[(CH3CO)2O][Pyridine] A}\]
The end product C of the following reaction is
\[\ce{C2H5NH2 ->[HNO2] A ->[PCl5] B ->[NH3][Alcohol] C}\]
Which of the following reagents is used in Mendius reduction reaction of alkyl cyanide?
The best reagent for converting 2–phenylpropanamide into 2-phenylpropanamine is ______.
Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is ______.
Which of the following amines can be prepared by Gabriel synthesis.
(i) Isobutyl amine
(ii) 2-Phenylethylamine
(iii) N-methylbenzylamine
(iv) Aniline
Which of the following reactions are correct?
(i)
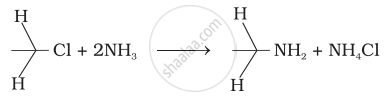
(ii)
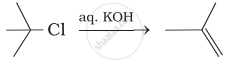
(iii)
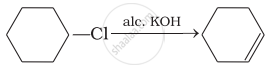
(iv)
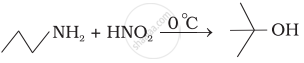
Write following conversions:
nitrobenzene `->` acetanilide
Write following conversions:
acetanilide `->` p-nitroaniline
Which of the following CANNOT be prepared by ammonolysis of alkyl halide?
Which of the following amines can be prepared by Gabriel phthalimide reaction?
- Phenyl methenamine
- N, N - Dimethylaniline
- N - Methyl aniline
- Benzenamine
Choose the correct order of the basic nature of the above amines.
The amine 'A' when treated with nitrous acid gives yellow oily substance. The amine A is ______.
Write a short note on the following:
Ammonolysis
Write short note on the following:
Ammonolysis
Write short notes on the following:
Ammonolysis
