Advertisements
Advertisements
प्रश्न
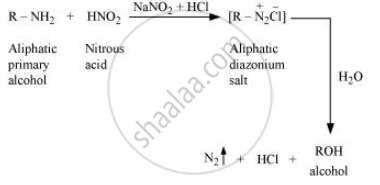
Write the reactions of aliphatic primary amines with nitrous acid.
उत्तर
Aliphatic primary amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) to form unstable aliphatic diazonium salts, which further produce alcohol and HCl with the evolution of N2 gas.
APPEARS IN
संबंधित प्रश्न
Accomplish the following conversions: Benzamide to toluene
Give the structures of A, B and C in the following reaction:
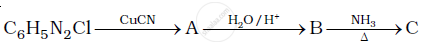
Give the structures of A, B and C in the following reactions:
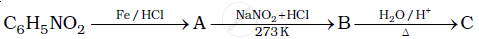
Give the structures of A, B and C in the following reaction:
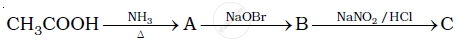
Give plausible explanation for each of the following :
Why are amines less acidic than alcohols of comparable molecular masses?
Explain Hoffmann’s exhaustive alkylation with suitable reactions.
Write reactions for the preparation of ethanamine using Gabriel phthalimide synthesis.
The end product C of the following reaction is
\[\ce{C2H5NH2 ->[HNO2] A ->[PCl5] B ->[NH3][Alcohol] C}\]
Identify the product obtained when benzyl chloride undergoes ammonolysis in presence of excess ammonia followed by the reaction with two moles of methyl iodide.
Which of the following does NOT give carbylamine test?
The source of nitrogen in Gabriel synthesis of amines is ______.
Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is ______.
Which of the following compounds is the weakest Brönsted base?
Which of the following methods of preparation of amines will give same number of carbon atoms in the chain of amines as in the reactant?
The Gabriels' phthalimide synthesis is used in the synthesis of
Write short note on the following:
Ammonolysis
Write short note on the following:
Ammonolysis
Write a short note on the following:
Ammonolysis
