Advertisements
Advertisements
प्रश्न
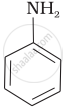
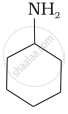
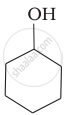
Which of the following compounds is the weakest Brönsted base?
पर्याय
उत्तर

Explanation:
Amines (i, ii) have stronger tendency to accept a proton and hence are stronger Bronsted bases than phenol (iii) and alcohol (iv). Since phenol is more acidic than alcohol, therefore, phenol (iii) has the least tendency to accept a proton and hence it is the weakest Bronsted base.
APPEARS IN
संबंधित प्रश्न
Illustrate the following reaction giving suitable example in each case:Gabriel phthalimide synthesis
Give the structures of A, B and C in the following reactions :
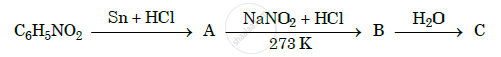
Answer in one sentence.
Which amide does produce ethanamine by Hofmann bromamide degradation reaction?
Write reactions for the preparation of ethanamine using Gabriel phthalimide synthesis.
Identify the product obtained when benzyl chloride undergoes ammonolysis in presence of excess ammonia followed by the reaction with two moles of methyl iodide.
In aqueous phase the order of basic strength of alkylamine is ______.
Which of the following compounds is obtained when quaternary ammonium hydroxide is strongly heated?
Hoffmann Bromamide Degradation reaction is shown by ______.
Write following conversions:
acetanilide `->` p-nitroaniline
Assertion: Hoffmann’s bromamide reaction is given by primary amines.
Reason: Primary amines are more basic than secondary amines.