Advertisements
Advertisements
Question
Give plausible explanation for each of the following :
Why are amines less acidic than alcohols of comparable molecular masses?
Solution 1
Amines undergo protonation to give amide ion.
`R- NH_2 -> R - bar(N) H + H^(+)`
Amide ion
Similarly, alcohol loses a proton to give alkoxide ion.
`R - OH -> R - bar(O) + H^(+)`
Alcohol Alkoxide ion
In an amide ion, the negative charge is on the N-atom whereas in alkoxide ion, the negative charge is on the O-atom. Since O is more electronegative than N, O can accommodate the negative charge more easily than N. As a result, the amide ion is less stable than the alkoxide ion. Hence, amines are less acidic than alcohols of comparable molecular masses
Solution 2
Loss of proton from an amine gives an amide ion while loss of a proton from alcohol give an alkoxide ion.
R—NH2—>R—NH– +H+
R—O —H—>R— O– +H+ .
Since O is more electronegative than N, so it wijl attract positive species more strongly in comparison to N. Thus, RO~ is more stable than RNH®. Thus, alcohols are more acidic than amines. Conversely, amines are less acidic than alcohols.
APPEARS IN
RELATED QUESTIONS
How is ethyl amine prepared from methyl iodide?
Give the structures of A, B and C in the following reaction:
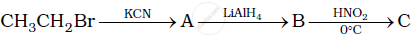
Write the reactions of aliphatic primary amines with nitrous acid.
Write the order of reactivity of alkyl halides with ammonia.
Identify compound 'B' in following series of reactions?
\[\ce{Acetonitrile ->[Na/alcohol] A ->[NaNO2/dil.HCI] B}\]
Identify the product obtained when benzyl chloride undergoes ammonolysis in presence of excess ammonia followed by the reaction with two moles of methyl iodide.
Which of the following amines exhibits maximum degree of intermolecular hydrogen bonding?
____________ can be prepared exclusively by Gabriel phthalimide synthesis.
Which of the following amines cannot be prepared by Gabriel phthalimide synthesis?
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): Alkyl halides are insoluble in water.
Reason (R): Alkyl halides have halogen attached to sp3 hybrid carbon.
Select the most appropriate answer from the options given below:
Amongst the given set of reactants, the most appropriate for preparing 2° amine is ______.
Among the following amines, the strongest Brönsted base is:
How will you carry out the following conversions?
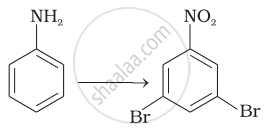
Which of the following amines can be prepared by Gabriel phthalimide reaction?
Amides can be converted into amines by the reaction named ______.
Identify A and B in the following reaction.
\[\ce{C6H5CH2Br ->[Alco.][KCN] A ->[Na/Ethanol][reduction] B}\]
Write a short note on Ammonolysis.
Write a short note on the following:
Ammonolysis
