Advertisements
Online Mock Tests
Chapters
2: Solutions
3: Electrochemistry
4: Chemical Kinetics
5: Surface Chemistry
6: General Principles and Processes of Isolation of Elements
7: The p-block Elements
8: The d-block and f-block Elements
9: Coordinate Compounds
10: Haloalkanes and Haloarenes
11: Alcohols, Phenols and Ethers
12: Aldehydes, Ketones and Carboxylic Acids
▶ 13: Amines
14: Biomolecules
15: Polymers
16: Chemistry in Everyday Life
Advertisements
Solutions for Chapter 13: Amines
Below listed, you can find solutions for Chapter 13 of CBSE, Karnataka Board PUC NCERT for Chemistry [English] Class 12.
NCERT solutions for Chemistry [English] Class 12 13 Amines Intext Questions [Pages 384 - 399]
Classify the following amine as primary, secondary or tertiary:

Primary
Secondary
Tertiary
Classify the following amine as primary, secondary or tertiary:
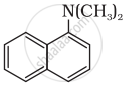
Primary
Secondary
Tertiary
Classify the following amine as primary, secondary or tertiary:
(C2H5)2CHNH2
Primary
Secondary
Tertiary
Classify the following amine as primary, secondary or tertiary:
(C2H5)2NH
Primary
Secondary
Tertiary
- Write structures of different isomeric amines corresponding to the molecular formula C4H11N.
- Write the IUPAC names of all the isomers.
- What type of isomerism is exhibited by different pairs of amines?
How will you convert Benzene into aniline?
How will you convert Benzene into N, N-dimethylaniline?
How will you convert Cl−(CH2)4−Cl into hexan-1, 6-diamine?
Arrange the following in increasing order of their basic strength:
C2H5NH2, C6H5NH2, NH3, C6H5CH2NH2 and (C2H5)2NH
Arrange the following in increasing order of their basic strength:
C2H5NH2, (C2H5)2NH, (C2H5)3N, C6H5NH2
Arrange the following in increasing order of their basic strength:
CH3NH2, (CH3)2NH, (CH3)3N, C6H5NH2, C6H5CH2NH2
Complete the following acid-base reaction and name the product:
\[\ce{CH3CH2CH2NH2 + HCl ->}\]
Complete the following acid-base reaction and name the product:
\[\ce{(C2H5)3N + HCl ->}\]
Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Write structures of different isomers corresponding to the molecular formula C3H9N. Write the IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
Convert aniline into 1, 3, 5-tribromobenzene.
Convert 3-Methylaniline into 3-nitrotoluene.
NCERT solutions for Chemistry [English] Class 12 13 Amines Exercises [Pages 400 - 402]
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)2CHNH2
Primary
Secondary
Tertiary
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3(CH2)2NH2
Primary
Secondary
Tertiary
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3NHCH(CH3)2
Primary
Secondary
Tertiary
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)3CNH2
Primary
Secondary
Tertiary
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
C6H5NHCH3
Primary
Secondary
Tertiary
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3CH2)2NCH3
Primary
Secondary
Tertiary
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
m−BrC6H4NH2
Primary
Secondary
Tertiary
Give one chemical test to distinguish between the following pair of compounds.
Methylamine and dimethylamine
Give one chemical test to distinguish between the following pair of compounds.
Secondary and tertiary amines
Give one chemical test to distinguish between the following pair of compounds.
Ethylamine and aniline
Give one chemical test to distinguish between the following pairs of compounds.
Aniline and benzylamine
Give one chemical test to distinguish between the following pair of compounds.
Aniline and N-methylaniline.
Account for the following:
pKb of aniline is more than that of methylamine.
Account for the following:
Ethylamine is soluble in water whereas aniline is not.
Account for the following:
Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
Account for the following:
Although the amino group is o, p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
Give reasons for the following:
Aniline does not undergo Friedel- Crafts reaction.
Account for the following:
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Account for the following:
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Arrange the following:
In decreasing order of the pKbvalues: C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Arrange the following:
In increasing order of basic strength: C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2
Arrange the following:
In increasing order of basic strength: Aniline, p-nitroaniline and p-toluidine
Arrange the following:
In increasing order of basic strength: C6H5NH2, C6H5NHCH3, C6H5CH2NH2.
Arrange the following:
In decreasing order of basic strength in gas phase: C2H5NH2, (C2H5)2NH, (C2H5)3N and NH3
Arrange the following:
In increasing order of boiling point: C2H5OH, (CH3)2NH, C2H5NH2
Arrange the following.
In increasing order of solubility in water, C6H5NH2, (C2H5)2NH, C2H5NH2
How will you convert Ethanoic acid into methanamine
How will you convert Hexanenitrile into 1-aminopentane
How will you convert Methanol to ethanoic acid
How will you convert Ethanamine into methanamine
How will you convert Ethanoic acid into propanoic acid
How will you convert Methanamine into ethanamine
How will you convert Nitromethane into dimethylamine
How will you convert Propanoic acid into ethanoic acid
Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Write short notes on the following Carbylamine reaction
Write a short note on Diazotisation
Write short notes on the following Hofmann’s bromamide reaction
Write short notes on the following Coupling reaction
Write a short note on Ammonolysis.
Write short notes on Acetylation
Write a short note on the following.
Gabriel phthalimide synthesis
Accomplish the following conversions Nitrobenzene to benzoic acid
Accomplish the following conversions - Benzene to m-bromophenol
Accomplish the following conversions - Benzoic acid to aniline
Accomplish the following conversions - Aniline to 2,4,6-tribromofluorobenzene
Accomplish the following conversions: Benzyl chloride to 2-phenylethanamine
Accomplish the following conversions - Chlorobenzene to p-chloroaniline
Accomplish the following conversions - Aniline to p-bromoaniline
Accomplish the following conversions: Benzamide to toluene
Accomplish the following conversions - Aniline to benzyl alcohol.
Give the structures of A, B and C in the following reaction:

Give the structures of A, B and C in the following reaction:
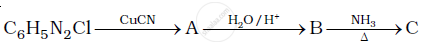
Give the structures of A, B and C in the following reaction:
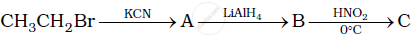
Give the structures of A, B and C in the following reactions:
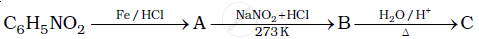
Give the structures of A, B and C in the following reaction:
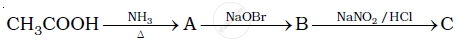
Give the structures of A, B and C in the following reaction.

An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B and C.
Complete the following reactions:
`C_6H_5NH_2 + (CH_3CO)_2O->`
Complete the following reactions:
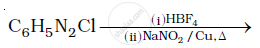
Complete the following reactions:
`C_6H_5NH_2 + Br_2(aq) ->`
Complete the following reactions:
`C_6H_5N_2Cl +C_2H_5OH->`
Complete the following reactions:
`C_6H_5N_2Cl+H_3PO_2+H_2O->`
Complete the following reactions:
`C_6H_5NH_2 +H_2SO_4(conc.) ->`
Complete the following reactions :-
`C_6H_5NH_2+CHCl_3 + alc.KOH ->`
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Write the reactions of aromatic with nitrous acid.
Write the reactions of aliphatic primary amines with nitrous acid.
Give plausible explanation for each of the following :
Why are amines less acidic than alcohols of comparable molecular masses?
Give plausible explanation for each of the following:
Why do primary amines have higher boiling point than tertiary amines?
Give plausible explanation for each of the following:
Why are aliphatic amines stronger bases than aromatic amines?
Solutions for 13: Amines
NCERT solutions for Chemistry [English] Class 12 chapter 13 - Amines
Shaalaa.com has the CBSE, Karnataka Board PUC Mathematics Chemistry [English] Class 12 CBSE, Karnataka Board PUC solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT solutions for Mathematics Chemistry [English] Class 12 CBSE, Karnataka Board PUC 13 (Amines) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. NCERT textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] Class 12 chapter 13 Amines are Introduction of Amines, Classification of Amines, Structure of Amines, Physical Properties of Amines, Nomenclature of Animes, Uses of Amines, Identification of Primary, Secondary and Tertiary Amines, Cyanides and Isocyanides, Introduction of Diazonium Salts, Method of Preparation of Diazonium Salts, Importance of Diazonium Salts in Synthesis of Aromatic Compounds, Preparation of Amines, Chemical Reactions of Amines - Basic Character of Amines, Chemical Reactions of Amines - Alkylation and Acylation, Chemical Reactions of Amines - Carbylamine Reaction, Chemical Reactions of Amines - Reaction with Nitrous Acid, Chemical Reactions of Amines - Reaction with Arylsulphonyl Chloride, Chemical Reactions of Amines - Electrophilic Substitution, Physical Properties of Diazonium Salts, Chemical Reaction of Diazonium Salts - Reactions Involving Displacement of Nitrogen, Chemical Reaction of Diazonium Salts - Reactions Involving Retention of Diazo Group, Organic Compounds Containing Nitrogen Numericals, Introduction of Amines, Classification of Amines, Structure of Amines, Physical Properties of Amines, Nomenclature of Animes, Uses of Amines, Identification of Primary, Secondary and Tertiary Amines, Cyanides and Isocyanides, Introduction of Diazonium Salts, Method of Preparation of Diazonium Salts, Importance of Diazonium Salts in Synthesis of Aromatic Compounds, Preparation of Amines, Chemical Reactions of Amines - Basic Character of Amines, Chemical Reactions of Amines - Alkylation and Acylation, Chemical Reactions of Amines - Carbylamine Reaction, Chemical Reactions of Amines - Reaction with Nitrous Acid, Chemical Reactions of Amines - Reaction with Arylsulphonyl Chloride, Chemical Reactions of Amines - Electrophilic Substitution, Physical Properties of Diazonium Salts, Chemical Reaction of Diazonium Salts - Reactions Involving Displacement of Nitrogen, Chemical Reaction of Diazonium Salts - Reactions Involving Retention of Diazo Group, Organic Compounds Containing Nitrogen Numericals.
Using NCERT Chemistry [English] Class 12 solutions Amines exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in NCERT Solutions are essential questions that can be asked in the final exam. Maximum CBSE, Karnataka Board PUC Chemistry [English] Class 12 students prefer NCERT Textbook Solutions to score more in exams.
Get the free view of Chapter 13, Amines Chemistry [English] Class 12 additional questions for Mathematics Chemistry [English] Class 12 CBSE, Karnataka Board PUC, and you can use Shaalaa.com to keep it handy for your exam preparation.
![NCERT solutions for Chemistry [English] Class 12 chapter 13 - Amines NCERT solutions for Chemistry [English] Class 12 chapter 13 - Amines - Shaalaa.com](/images/9788174506481-chemistry-english-class-12_6:a55896f658974483bc7dc0613af00ce2.jpg)
