Advertisements
Advertisements
Question
Give one chemical test to distinguish between the following pair of compounds.
Ethylamine and aniline
Solution
Ethylamine is a primary aliphatic amine, while aniline is a primary aromatic amine. They can be differentiated by azo dye test.
Azo dye test: In this, aromatic primary amine reacts with HNO2 (NaNO2 + dil. HCl) at 273 – 278 K and then reacts with alkaline solution of 2-naphthol (β-naphthol) to give a deep yellow, orange or red colored dye.
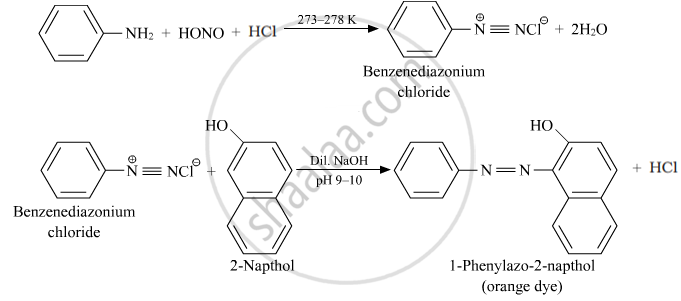
Aliphatic primary amines under the above conditions rapidly release nitrogen gas with the formation of primary alcohols, i.e., the solution remains transparent.
APPEARS IN
RELATED QUESTIONS
Convert aniline into 1, 3, 5-tribromobenzene.
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3NHCH(CH3)2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
C6H5NHCH3
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
m−BrC6H4NH2
Give one chemical test to distinguish between the following pair of compounds.
Aniline and benzylamine
How will you convert methanol to ethanoic acid?
How will you convert ethanoic acid into propanoic acid?
How will you convert nitromethane into dimethylamine?
How will you convert propanoic acid into ethanoic acid?
Accomplish the following conversions - Aniline to benzyl alcohol.
Complete the following reactions:
Do the following conversions in not more than two steps :
Propanone to Propene
Write the structure of 2,4-dinitrochlorobenzene
What is the action of p-toluenesulphonychloride on ethylamine and diethylamine?
Write the IUPAC name of the given compound :
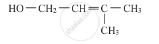
The following amine is called as:
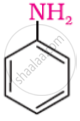
Predict the product 'A' in the following reaction.
