Advertisements
Advertisements
Question
Give one chemical test to distinguish between the following pair of compounds.
Secondary and tertiary amines
Solution
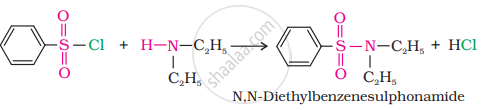
Secondary and tertiary amines can be distinguished by allowing them to react with Hinsberg’s reagent (benzenesulphonyl chloride, C6H5SO2Cl).
Secondary amines react with Hinsberg’s reagent to form a product that is insoluble in an alkali. For example, N, N−diethylamine reacts with Hinsberg’s reagent to form N, N−diethylbenzenesulphonamide, which is insoluble in an alkali. Tertiary amines, however, do not react with Hinsberg’s reagent.
APPEARS IN
RELATED QUESTIONS
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
C6H5NHCH3
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3CH2)2NCH3
Give one chemical test to distinguish between the following pairs of compounds.
Aniline and benzylamine
Account for the following:
pKb of aniline is more than that of methylamine.
How will you convert Hexanenitrile into 1-aminopentane
How will you convert Methanol to ethanoic acid
How will you convert Ethanamine into methanamine
Accomplish the following conversions - Chlorobenzene to p-chloroaniline
Complete the following reactions:
`C_6H_5NH_2 +H_2SO_4(conc.) ->`
Complete the following reactions:
`C_6H_5NH_2 + Br_2(aq) ->`
Give reasons Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline
Do the following conversions in not more than two steps :
Ethyl benzene to Benzoic acid
Do the following conversions in not more than two steps :
Propanone to Propene
Using IUPAC norms write the formula of Hexaamminecobalt (III) sulphate.
The following amine is called as:
An organic compound (A) with molecular formula C3H7NO on heating with Br2 and KOH forms a compound (B). Compound (B) on heating with CHCl3 and alcoholic KOH produces a foul-smelling compound (C) and on reacting with C6H5SO2Cl forms a compound (D) which is soluble in alkali. Write the structure of (A), (B), (C) and (D).
Do the following conversions in not more than two steps:
\[\begin{array}{cc}
\ce{CH3CN to CH3 - C - CH3}\\
\phantom{...........}||\\
\phantom{...........}\ce{O}
\end{array}\]
Predict the product 'A' in the following reaction.
\[\ce{\underset{(Acetyl chloride)}{CH3COCl}->[H2][Pd-BaSO4]A + HCl}\]
