Advertisements
Advertisements
Question
How will you convert methanol to ethanoic acid?
Solution
\[\ce{\underset{Methanol}{CH3OH} ->[PCl5] \underset{Chloromethane}{CH3Cl} ->[KCN (alc.)] \underset{Ethanenitrile}{CH3CN} ->[H3O+] \underset{Ethanoic acid}{CH3COOH} }\]
APPEARS IN
RELATED QUESTIONS
Convert 3-Methylaniline into 3-nitrotoluene.
Convert aniline into 1, 3, 5-tribromobenzene.
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3(CH2)2NH2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3NHCH(CH3)2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3CH2)2NCH3
Give one chemical test to distinguish between the following pair of compounds.
Methylamine and dimethylamine
Account for the following:
pKb of aniline is more than that of methylamine.
How will you convert hexanenitrile into 1-aminopentane?
How will you convert Ethanamine into methanamine
How will you convert Ethanoic acid into propanoic acid
How will you convert Methanamine into ethanamine
Accomplish the following conversions - Aniline to 2,4,6-tribromofluorobenzene
Accomplish the following conversions - Chlorobenzene to p-chloroaniline
Complete the following reactions:
`C_6H_5N_2Cl +C_2H_5OH->`
Complete the following reactions:
`C_6H_5NH_2 + Br_2(aq) ->`
Complete the following reactions:
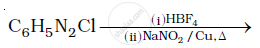
Give reasons Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline
Using IUPAC norms write the formula of Hexaamminecobalt (III) sulphate.
The following amine is called as:
Predict the product 'A' in the following reaction.
\[\ce{\underset{(Acetyl chloride)}{CH3COCl}->[H2][Pd-BaSO4]A + HCl}\]
