Advertisements
Advertisements
Question
Write a short note on Ammonolysis.
Solution
When an alkyl or benzyl halide is allowed to react with an ethanolic solution of ammonia, it undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino (−NH2) group. This process of cleavage of the carbon-halogen bond is known as ammonolysis.
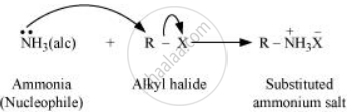
Amine is obtained when this substituted ammonium salt is treated with a strong base such as sodium hydroxide.
\[\ce{R - \overset{+}{N}H3 \overset{-}{X} + NaOH -> \underset{Amine}{R - NH}_2 + H2O + NaX}\]
Though primary amine is produced as the major product, this process produces a mixture of primary, secondary and tertiary amines, and also a quaternary ammonium salt as shown.
\[\ce{\underset{(1^\circ)}{RN}H2 ->[RX] \underset{(2^\circ)}{R2N}H ->[RX] \underset{(3^\circ)}{R3}N ->[RX] \underset{ammonium salt}{\underset{Quaternary}{R4 \overset{+}{N}\overset{-}{X}}}}\]
APPEARS IN
RELATED QUESTIONS
Identify the weakest base amongst the following :
(a) p- methoxyaniline
(b) o-toluidine
(c) benzene - 1, 4 - diamine
(d) 4 - aminobenzoic acid
Give reasons for the following: (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
Give reason for the following:
Primary amines have higher boiling point than tertiary amines.
Arrange the following in increasing order of their basic strength :

- Write structures of different isomeric amines corresponding to the molecular formula C4H11N.
- Write the IUPAC names of all the isomers.
- What type of isomerism is exhibited by different pairs of amines?
How will you convert Benzene into aniline?
How will you convert Benzene into N, N-dimethylaniline?
Complete the following acid-base reaction and name the product:
\[\ce{(C2H5)3N + HCl ->}\]
Accomplish the following conversions - Aniline to p-bromoaniline
Give reason (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
Arrange the following in the increasing order of their pKb values:
C6H5NH2, C2H5NH2, C6H5NHCH3
Arrange the following in increasing order of basic strength :
C6H5NH2, C6H5NHCH3, C6H5N(CH3)2
Choose the most correct option.
Which one of the following compounds has the highest boiling point?
The CORRECT decreasing order of boiling points is:
Among the following isomeric amines, an amine having highest boiling point is:
Account for the following:
N-ethylethanamine boils at 329.3 K and butanamine boils at 350.8 K, although both are isomeric in nature.
The hydrogen bond is shortest in
Arrange the following in increasing order of their boiling point:
C2H5OH, C2H5NH2, (C2H5)3N
