Advertisements
Advertisements
Question
An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B and C.
Solution 1
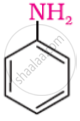
It is given that compound ‘C’ having the molecular formula, C6H7N is formed by heating compound ‘B’ with Br2 and KOH. This is a Hoffmann bromamide degradation reaction. Therefore, compound ‘B’ is an amide and compound ‘C’ is an amine. The only amine having the molecular formula, C6H7N is aniline, (C6H5NH2).
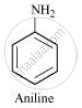
Therefore, compound ‘B’ (from which ’C’ is formed) must be benzamide, (C6H5CONH2).
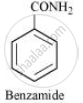
Further, benzamide is formed by heating compound ‘A’ with aqueous ammonia. Therefore, compound ‘A’ must be benzoic acid.
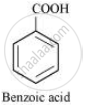
The given reactions can be explained with the help of the following equations:

Solution 2
Since the compound ‘C’ with molecular formula C6H7N is formed from compound ‘B’ on treatment with Br2 KOH, therefore, compound ‘B’ must be an amide and ‘C’ must be an amine.
The only amine having the molecular formula C6H7N, i. e., C6H5NH2 is aniline.
Since ‘C’ is aniline, therefore, die amide from which it is formed must be benzamide (C6H5CONH2). Thus, compound‘B’is benzamide. Since compound ‘B’ is formed from compound ‘A’ with aqueous ammonia and heating, therefore, compound ‘A’ must be benzoic acid.
APPEARS IN
RELATED QUESTIONS
Which among the following molecular formulae represents urotropine?
(a) C6H12N4
(b) C6H24H4
(c) C6H12N4O2
(d) C6H24N4O2
Convert 3-Methylaniline into 3-nitrotoluene.
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
CH3NHCH(CH3)2
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
(CH3)3CNH2
Give one chemical test to distinguish between the following pair of compounds.
Methylamine and dimethylamine
Give one chemical test to distinguish between the following pairs of compounds.
Aniline and benzylamine
How will you convert Methanol to ethanoic acid
How will you convert Ethanamine into methanamine
How will you convert Methanamine into ethanamine
Accomplish the following conversions - Chlorobenzene to p-chloroaniline
Complete the following reactions:
`C_6H_5NH_2 + (CH_3CO)_2O->`
How are ethylamine and ethyl methyl amine distinguished by using nitrous acid?
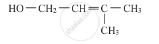
Write the IUPAC name of the given compound :
Using IUPAC norms write the formula of Hexaamminecobalt (III) sulphate.
The following amine is called as:
An organic compound (A) with molecular formula C3H7NO on heating with Br2 and KOH forms a compound (B). Compound (B) on heating with CHCl3 and alcoholic KOH produces a foul-smelling compound (C) and on reacting with C6H5SO2Cl forms a compound (D) which is soluble in alkali. Write the structure of (A), (B), (C) and (D).
