Advertisements
Advertisements
Question
Account for the following:
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Solution 1
Diazonium salts of aromatic amines are more stable than those of aliphatic amines:
The diazonium ion undergoes resonance as shown below:
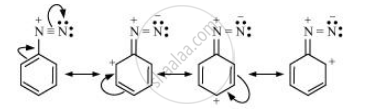
This resonance accounts for the stability of the diazonium ion. Hence, diazonium salts of aromatic amines are more stable than those of aliphatic amines.
shaalaa.com
Solution 2
The diazonium salts of aromatic amines are more stable than those of aliphapic amines due to dispersal of the positive charge on benzene ring as a result of resonance.
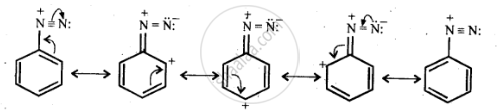
shaalaa.com
Is there an error in this question or solution?
