Advertisements
Advertisements
Question
Arrange the following:
In decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Solution
In C2H5NH2, only one −C2H5 group is present while in (C2H5)2NH, two −C2H5 groups are present. Thus, the +I effect is more in (C2H5)2NH than in C2H5NH2. Therefore, the electron density over the N-atom is more in (C2H5)2NH than in C2H5NH2. Hence, (C2H5)2NH is more basic than C2H5NH2.
Also, both C6H5NHCH3 and C6H5NH2 are less basic than (C2H5)2NH and C2H5NH2 due to the delocalization of the lone pair in the former two. Further, among C6H5NHCH3 and C6H5NH2, the former will be more basic due to the +I effect of −CH3 group. Hence, the order of increasing basicity of the given compounds is as follows:
C6H5NH2 < C6H5NHCH3 < C2H5NH2 < (C2H5)2NH
We know that the higher the basic strength, the lower the pKb values.
C6H5NH2 > C6H5NHCH3 > C2H5NH2 > (C2H5)2NH
APPEARS IN
RELATED QUESTIONS
Write the chemical equations involved when aniline is treated with the following reagents: HCI
Arrange the following:
In increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2
Arrange the following:
In increasing order of basic strength: Aniline, p-nitroaniline and p-toluidine
Arrange the following:
In increasing order of basic strength: C6H5NH2, C6H5NHCH3, C6H5CH2NH2.
Write short notes on the following Carbylamine reaction
Write the structures of the main products of the following reactions:
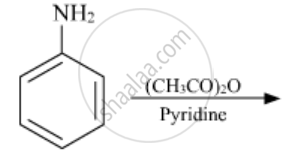
Write the structures of the main products of the following reactions:
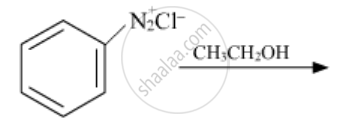
The following reaction takes place in the presence of:
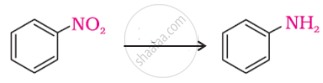
Arrange the following in decreasing order of their basic strength:
C6H5NH2, C2H5NH2, (C2H5)2NH2, NH3
Account for the following:
Acylation of aniline is carried out in the presence of pyridine.
Which of the following statement is true about methyl amine?
Which of the following is most basic?
Account for the following:
Arrange the following compounds in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
Which of the following compound cannot be produced if 1-propane amine is treated with NaNO2 and HCl?
State one reason for the following:
Alkylamine is soluble in water, whereas arylamine is insoluble in water.
The correct order of the increasing basic nature of Ammonia, Methylamine and Aniline is:
