Advertisements
Advertisements
Question
Account for the following:
Acylation of aniline is carried out in the presence of pyridine.
Solution
During the acylation of aniline, stronger base pyridine is added. This done in order to remove the HCl so formed during the reaction and to shift the equilibrium to the right hand side.
APPEARS IN
RELATED QUESTIONS
Why does NH3 act as a Lewis base?
Arrange the following:
In decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Write the structures of the main products of the following reactions:

Write the structures of the main products of the following reactions:
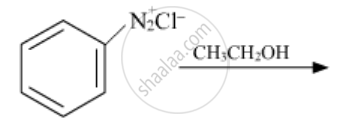
Arrange the following in decreasing order of their basic strength:
C6H5NH2, C2H5NH2, (C2H5)2NH2, NH3
The correct decreasing order of basic strength of the following species is ______.
\[\ce{H2O, NH3, OH-, NH^{-}2}\]
By the presence of a halogen atom in the ring, what is the effect of this on basic property of aniline?
What is the correct decreasing order of the basic character of the three amines and ammonia?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
The correct order of the increasing basic nature of Ammonia, Methylamine and Aniline is:
