Advertisements
Advertisements
Question
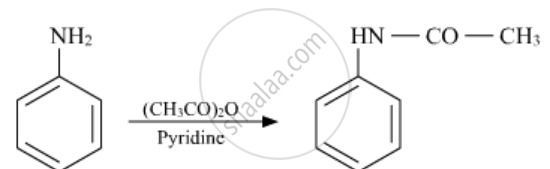
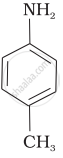
Write the structures of the main products of the following reactions:
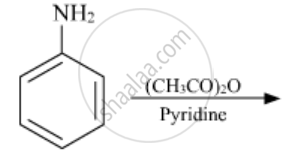
Solution
APPEARS IN
RELATED QUESTIONS
Arrange the following in increasing order of their basic strength in aqueous solution:
\[\ce{CH3NH2, (CH3)3N, (CH3)2NH}\]
Write the chemical equations involved when aniline is treated with the following reagents: HCI
Arrange the following:
In increasing order of basic strength: C6H5NH2, C6H5NHCH3, C6H5CH2NH2.
Write the structures of the main products of the following reactions:

The correct increasing order of basic strength for the following compounds is ______.
(I)
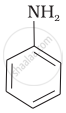
(II)
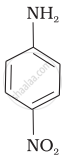
(III)
When ethanol is mixed with ammonia and passed over alumina the compound formed is which compound?
Which of the following statement is true about methyl amine?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
State one reason for the following:
Alkylamine is soluble in water, whereas arylamine is insoluble in water.
The correct order of the increasing basic nature of Ammonia, Methylamine and Aniline is:
