Advertisements
Advertisements
Question
The correct increasing order of basic strength for the following compounds is ______.
(I)
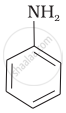
(II)
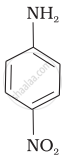
(III)
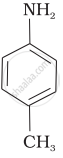
Options
II < III < I
III < I < II
III < II < I
II < I < III
Solution
The correct increasing order of basic strength for the following compounds is II < I < III.
Explanation:
The greater the electron density towards the ring, the greater its basic strength. The electron-withdrawing group reduces basic strength, whereas the electron-donating group increases basic strength.
APPEARS IN
RELATED QUESTIONS
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
Write the chemical equations involved when aniline is treated with the following reagents: HCI
Arrange the following:
In increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2
Write the structures of the main products of the following reactions:
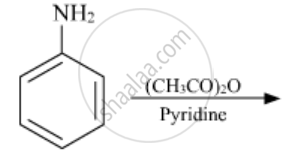
The most reactive amine towards dilute hydrochloric acid is:
Explain why \[\ce{MeNH2}\] is stronger base than \[\ce{MeOH}\]?
Which of the following is most basic?
What is the characteristic smell of liquid amines?
Give reasons for the following observation:
pKb of aniline is lower than the m-nitroaniline.
Account for the following:
Arrange the following compounds in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
