Advertisements
Advertisements
Question
Give reasons for the following observation:
pKb of aniline is lower than the m-nitroaniline.
Solution
pKb of aniline is lower than the m-nitro aniline.The basic strength of aniline is more than m-nitroaniline. pKb value is inversely proportional to basic strength. The presence of an Electron withdrawing group decreases basic strength.
APPEARS IN
RELATED QUESTIONS
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
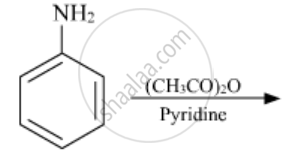
Write the structures of the main products of the following reactions:
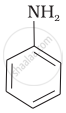
The correct increasing order of basic strength for the following compounds is ______.
(I)
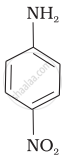
(II)
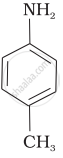
(III)
The most reactive amine towards dilute hydrochloric acid is:
When methyl iodide is heated with ammonia, what is the product obtained?
When ethanol is mixed with ammonia and passed over alumina the compound formed is which compound?
A Solution of methyl amine shows which type of property with litmus paper?
Which of the following is most basic?
State one reason for the following:
Alkylamine is soluble in water, whereas arylamine is insoluble in water.
The correct order of the increasing basic nature of Ammonia, Methylamine and Aniline is:
