Advertisements
Advertisements
प्रश्न
Give reasons for the following observation:
pKb of aniline is lower than the m-nitroaniline.
उत्तर
pKb of aniline is lower than the m-nitro aniline.The basic strength of aniline is more than m-nitroaniline. pKb value is inversely proportional to basic strength. The presence of an Electron withdrawing group decreases basic strength.
APPEARS IN
संबंधित प्रश्न
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
Write the structures of main products when aniline reacts with the following reagents : HCl
Arrange the following:
In increasing order of basic strength:
Aniline, p-nitroaniline and p-toluidine
The correct increasing order of basic strength for the following compounds is ______.
(I)
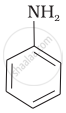
(II)
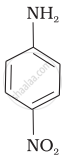
(III)
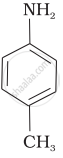
The correct decreasing order of basic strength of the following species is ______.
\[\ce{H2O, NH3, OH-, NH^{-}2}\]
When methyl iodide is heated with ammonia, what is the product obtained?
By the presence of a halogen atom in the ring, what is the effect of this on basic property of aniline?
What is the characteristic smell of liquid amines?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
State one reason for the following:
Alkylamine is soluble in water, whereas arylamine is insoluble in water.
