Advertisements
Advertisements
प्रश्न
The correct increasing order of basic strength for the following compounds is ______.
(I)
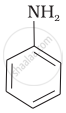
(II)
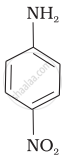
(III)
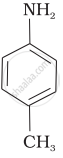
विकल्प
II < III < I
III < I < II
III < II < I
II < I < III
उत्तर
The correct increasing order of basic strength for the following compounds is II < I < III.
Explanation:
The greater the electron density towards the ring, the greater its basic strength. The electron-withdrawing group reduces basic strength, whereas the electron-donating group increases basic strength.
APPEARS IN
संबंधित प्रश्न
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
Arrange the following:
In decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Arrange the following:
In increasing order of basic strength:
Aniline, p-nitroaniline and p-toluidine
Write the structures of the main products of the following reactions:

Arrange the following in decreasing order of their basic strength:
C6H5NH2, C2H5NH2, (C2H5)2NH2, NH3
When methyl iodide is heated with ammonia, what is the product obtained?
Give reasons for the following observation:
pKb of aniline is lower than the m-nitroaniline.
Account for the following:
Arrange the following compounds in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
Which of the following compound cannot be produced if 1-propane amine is treated with NaNO2 and HCl?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
