Advertisements
Advertisements
Question
Explain why \[\ce{MeNH2}\] is stronger base than \[\ce{MeOH}\]?
Solution
As the electronegative of oxygen is more than the electronegativity of a nitrogen atom, the \[\ce{O - H}\] bond is more polar than the \[\ce{N - H}\] bond, therefore \[\ce{MeOH}\] is stronger acod than \[\ce{MeNH2}\] or \[\ce{MeNH2}\] 2 is stronger base than \[\ce{MeOH}\].
APPEARS IN
RELATED QUESTIONS
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
Write the structures of the main products of the following reactions:
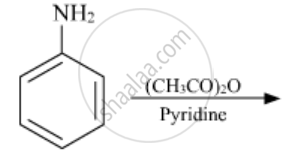
Write the structures of the main products of the following reactions:

Write the structures of the main products of the following reactions:
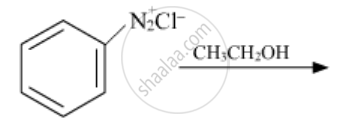
When methyl iodide is heated with ammonia, what is the product obtained?
Which of the following is most basic?
By the presence of a halogen atom in the ring, what is the effect of this on basic property of aniline?
Account for the following:
Arrange the following compounds in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
Which of the following compound cannot be produced if 1-propane amine is treated with NaNO2 and HCl?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
