Advertisements
Advertisements
प्रश्न
Explain why \[\ce{MeNH2}\] is stronger base than \[\ce{MeOH}\]?
उत्तर
As the electronegative of oxygen is more than the electronegativity of a nitrogen atom, the \[\ce{O - H}\] bond is more polar than the \[\ce{N - H}\] bond, therefore \[\ce{MeOH}\] is stronger acod than \[\ce{MeNH2}\] or \[\ce{MeNH2}\] 2 is stronger base than \[\ce{MeOH}\].
APPEARS IN
संबंधित प्रश्न
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
Arrange the following:
In decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Write the structures of the main products of the following reactions:
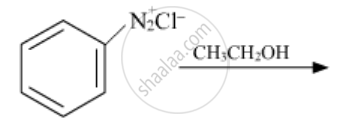
The following reaction takes place in the presence of:
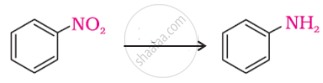
The correct increasing order of basic strength for the following compounds is ______.
(I)
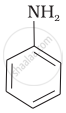
(II)
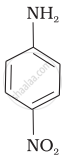
(III)
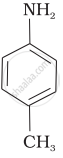
The most reactive amine towards dilute hydrochloric acid is:
The correct decreasing order of basic strength of the following species is ______.
\[\ce{H2O, NH3, OH-, NH^{-}2}\]
Which of the following statement is true about methyl amine?
What is the characteristic smell of liquid amines?
Give reasons for the following observation:
pKb of aniline is lower than the m-nitroaniline.
