Advertisements
Advertisements
प्रश्न
The most reactive amine towards dilute hydrochloric acid is:
पर्याय
\[\ce{CH3 - NH2}\]
\[\begin{array}{cc}
\ce{H3C}\\
\phantom{......}\backslash\\
\phantom{...........}\ce{NH}\\
\phantom{......}/\\
\phantom{}\ce{H3C}\\
\end{array}\]\[\begin{array}{cc}
\ce{H3C}\\
\phantom{......}\backslash\\
\phantom{.................}\ce{NH - CH3}\phantom{}\\
\phantom{......}/\\
\phantom{}\ce{H3C}\\
\end{array}\]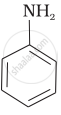
उत्तर
\[\begin{array}{cc}
\ce{H3C}\\
\phantom{......}\backslash\\
\phantom{...........}\ce{NH}\\
\phantom{......}/\\
\phantom{}\ce{H3C}\\
\end{array}\]
Explanation:
The greater will be the strength of base, the greater will be its reactivity towards dilute \[\ce{HCl}\]. Hence, \[\ce{(CH3)2NH}\] has the highest basic strength as it has the highest reactivity.
APPEARS IN
संबंधित प्रश्न
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
Write the structures of main products when aniline reacts with the following reagents : HCl
Write the chemical equations involved when aniline is treated with the following reagents: HCI
Arrange the following:
In increasing order of basic strength: Aniline, p-nitroaniline and p-toluidine
Explain why \[\ce{MeNH2}\] is stronger base than \[\ce{MeOH}\]?
A Solution of methyl amine shows which type of property with litmus paper?
Which of the following is most basic?
Among the following, which has the highest value of pKb?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
The correct order of the increasing basic nature of Ammonia, Methylamine and Aniline is:
