Advertisements
Advertisements
प्रश्न
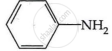
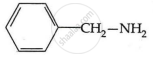
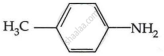
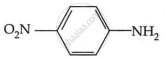
Among the following, which has the highest value of pKb?
पर्याय
उत्तर
Explanation:
The pKb number is proportional to the basic strength. The electron density on the nitrogen atom drops due to the presence of the electron-withdrawing NO2 group.
As a result, phenylmethanamine is the least basic. Thus, it will have the highest pKb value.
APPEARS IN
संबंधित प्रश्न
Write the chemical equations involved when aniline is treated with the following reagents: HCI
Arrange the following:
In increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2
Arrange the following:
In increasing order of basic strength: Aniline, p-nitroaniline and p-toluidine
Arrange the following:
In decreasing order of basic strength in gas phase: C2H5NH2, (C2H5)2NH, (C2H5)3N and NH3
Write short notes on the following Carbylamine reaction
The correct decreasing order of basic strength of the following species is ______.
\[\ce{H2O, NH3, OH-, NH^{-}2}\]
Explain why \[\ce{MeNH2}\] is stronger base than \[\ce{MeOH}\]?
A Solution of methyl amine shows which type of property with litmus paper?
What is the characteristic smell of liquid amines?
Give reasons for the following observation:
pKb of aniline is lower than the m-nitroaniline.