Advertisements
Advertisements
Question
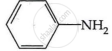
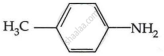
Among the following, which has the highest value of pKb?
Options
Solution
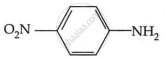
Explanation:
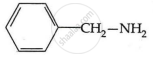
The pKb number is proportional to the basic strength. The electron density on the nitrogen atom drops due to the presence of the electron-withdrawing NO2 group.
As a result, phenylmethanamine is the least basic. Thus, it will have the highest pKb value.
APPEARS IN
RELATED QUESTIONS
Arrange the following in increasing order of their basic strength in aqueous solution:
\[\ce{CH3NH2, (CH3)3N, (CH3)2NH}\]
Write the structures of main products when aniline reacts with the following reagents : HCl
Arrange the following:
In decreasing order of the pKbvalues: C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Write short notes on the following Carbylamine reaction
The correct decreasing order of basic strength of the following species is ______.
\[\ce{H2O, NH3, OH-, NH^{-}2}\]
Explain why \[\ce{MeNH2}\] is stronger base than \[\ce{MeOH}\]?
Account for the following:
Acylation of aniline is carried out in the presence of pyridine.
A Solution of methyl amine shows which type of property with litmus paper?
What is the characteristic smell of liquid amines?
The correct order of the increasing basic nature of Ammonia, Methylamine and Aniline is: