Advertisements
Advertisements
Question
Methylamine reacts with \[\ce{HNO2}\] to form ______.
Options
\[\ce{CH3 - O - N = O}\]
\[\ce{CH3 - O - CH3}\]
\[\ce{CH3OH}\]
\[\ce{CH3CHO}\]
Solution
Methylamine reacts with \[\ce{HNO2}\] to form \[\ce{CH3OH}\].
Explanation:
Methyl amine reacts with \[\ce{HNO3}\] form methanol with release of nitrogen gas and water as side products.
\[\ce{CH3NH2 + HNO3 ->[NanO2 + HCl] CH3 - N2+Cl- ->[H2O] CH3OH + N2 + HCl}\]
APPEARS IN
RELATED QUESTIONS
What is Stephen reaction?
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{CH3CH2I ->[NaCN] A ->[OH^-][Partial hydrolysis] B ->[NaOH + Br2] C}\]
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{CH3NH2 ->[CH3Br] A ->[CH3COCl] B ->[B2H6] C}\]
Identify compounds A, B and C in the following sequence of reaction.
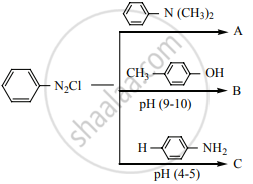
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{CH3CH2NC ->[HgO] A ->[H2O] B ->[i) NaNO2/HCl][ii) H2O] C}\]
Predict A, B, C and D for the following reaction.
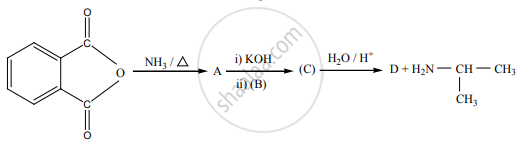
Which of the following species are involved in the carbylamine test?
(i) \[\ce{R - NC}\]
(ii) \[\ce{CHCl3}\]
(iii) \[\ce{COCl2}\]
(iv) \[\ce{NaNO2 + HCl}\]
Among the following, which the strongest base?
Alkyl cyanides can be obtained from primary acid amides using ______.
Write short note on:
Ammonolysis
