Advertisements
Advertisements
Question
Arrange the following:
In increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2
Solution
C6H5N(CH3)2 is more basic than C6H5NH2 due to the presence of the +I effect of two −CH3 groups in C6H5N(CH3)2. Further, CH3NH2 contains one −CH3 group while (C2H5)2NH contains two −C2H5groups. Thus, (C2H5)2NH is more basic than C2H5NH2.
Now, C6H5N(CH3)2 is less basic than CH3NH2 because of the −R effect of −C6H5 group.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
C6H5NH2 < C6H5N(CH3)2 < CH3NH2 < (C2H5)2NH
APPEARS IN
RELATED QUESTIONS
Arrange the following in increasing order of their basic strength in aqueous solution:
\[\ce{CH3NH2, (CH3)3N, (CH3)2NH}\]
Write the chemical equations involved when aniline is treated with the following reagents: HCI
Why does NH3 act as a Lewis base?
Arrange the following:
In decreasing order of basic strength in gas phase: C2H5NH2, (C2H5)2NH, (C2H5)3N and NH3
Write short notes on the following Carbylamine reaction
Write the structures of the main products of the following reactions:

Write the structures of the main products of the following reactions:
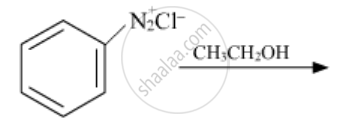
Arrange the following in decreasing order of their basic strength:
C6H5NH2, C2H5NH2, (C2H5)2NH2, NH3
The correct increasing order of basic strength for the following compounds is ______.
(I)
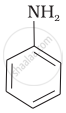
(II)
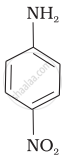
(III)
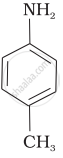
When ethanol is mixed with ammonia and passed over alumina the compound formed is which compound?
Which of the following statement is true about methyl amine?
Which of the following is most basic?
By the presence of a halogen atom in the ring, what is the effect of this on basic property of aniline?
Account for the following:
Arrange the following compounds in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
State one reason for the following:
Alkylamine is soluble in water, whereas arylamine is insoluble in water.
