Advertisements
Advertisements
प्रश्न
Arrange the following in increasing order of their basic strength in aqueous solution:
\[\ce{CH3NH2, (CH3)3N, (CH3)2NH}\]
उत्तर
\[\ce{(CH3)3N < CH3NH2 < (CH3)2NH}\]
संबंधित प्रश्न
Why does NH3 act as a Lewis base?
Arrange the following:
In decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Write the structures of the main products of the following reactions:
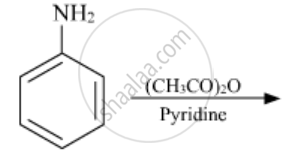
The following reaction takes place in the presence of:
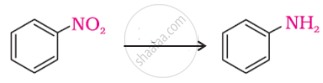
The most reactive amine towards dilute hydrochloric acid is:
When ethanol is mixed with ammonia and passed over alumina the compound formed is which compound?
Which of the following statement is true about methyl amine?
Which of the following is most basic?
What is the characteristic smell of liquid amines?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
