Advertisements
Advertisements
प्रश्न
Arrange the following in increasing order of their basic strength in aqueous solution:
\[\ce{CH3NH2, (CH3)3N, (CH3)2NH}\]
उत्तर
\[\ce{(CH3)3N < CH3NH2 < (CH3)2NH}\]
संबंधित प्रश्न
Why does NH3 act as a Lewis base?
Write short notes on the following Carbylamine reaction
The correct increasing order of basic strength for the following compounds is ______.
(I)
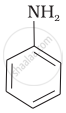
(II)
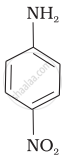
(III)
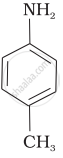
The most reactive amine towards dilute hydrochloric acid is:
A Solution of methyl amine shows which type of property with litmus paper?
Which of the following is most basic?
What is the characteristic smell of liquid amines?
Account for the following:
Arrange the following compounds in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
Among the following, which has the highest value of pKb?
State one reason for the following:
Alkylamine is soluble in water, whereas arylamine is insoluble in water.
