Advertisements
Advertisements
प्रश्न
State one reason for the following:
Alkylamine is soluble in water, whereas arylamine is insoluble in water.
उत्तर
Alkylamine is soluble in water because it has the potential to form hydrogen bonds with water. On the other hand, arylamines contain benzene, which is very hydrophobic. As a result, arylamines cannot be dissolved in water.
APPEARS IN
संबंधित प्रश्न
Arrange the following in increasing order of their basic strength in aqueous solution:
\[\ce{CH3NH2, (CH3)3N, (CH3)2NH}\]
Arrange the following:
In decreasing order of the pKb values:
C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
Arrange the following:
In increasing order of basic strength:
C6H5NH2, C6H5NHCH3, C6H5CH2NH2
The following reaction takes place in the presence of:
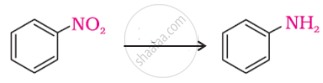
Explain why \[\ce{MeNH2}\] is stronger base than \[\ce{MeOH}\]?
Account for the following:
Acylation of aniline is carried out in the presence of pyridine.
What is the characteristic smell of liquid amines?
What is the correct decreasing order of the basic character of the three amines and ammonia?
Which of the following compound cannot be produced if 1-propane amine is treated with NaNO2 and HCl?
Among the following, which has the highest value of pKb?
