Advertisements
Advertisements
प्रश्न
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
विकल्प
Assertion and reason both are true, reason is correct explanation of assertion.
Assertion and reason both are true but reason is not the correct explanation of assertion.
Assertion is true, reason is false.
Assertion is false, reason is true.
उत्तर
Assertion and reason both are true but reason is not the correct explanation of assertion.
Explanation:
For complexes of MX6 and MX5L type, different geometric arrangements of the ligands is not possible.
MA4B2, M(AA)2B2 and MA3B3 type of complexes are the complexes with coordination number 6 which show geometrical isomerism.
APPEARS IN
संबंधित प्रश्न
The pair [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 exhibits ____________ isomerism
Which one of the following complexes is not expected to exhibit isomerism?
Consider the two complexes given below:
\[\ce{\underset{(I)}{[Co(NH3)5SO4]Br}}\] and \[\ce{\underset{(II)}{[Co(NH3)5Br]SO4}}\]
I and II are ____________ isomers.
\[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2 Cl(NO2)]}\] is ______.
The relationship between compound (i) and (ii) is
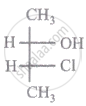 |
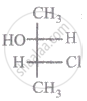 |
| (i) | (ii) |
Give cis isomer of [Co(NH3)4Cl2]⊕.
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [Pt(NH3)(H2O)Cl2]
Explain the geometrical isomerism of the octahedral complex of the type [M(AA)2B2]n± with a suitable example.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Co(NH3)5 [ONO]Cl2 and [Co(NH3)5(NO2)]Cl2}\]
Which one of the following complex ions has geometrical isomers?
