Advertisements
Advertisements
प्रश्न
Calculate magnetic moment of thorium (Z=90). Is this element diamagnetic or paramagnetic?
उत्तर
The electronic configuration of thorium is [Rn] 5f0 6d2 7s2.
the condensed electronic configuration for its divalent cation will be
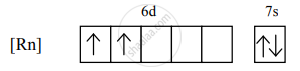
There are 2 unpaired electrons, so n = 2.
`therefore mu = sqrt(2 (2 + 2)) = sqrt8` BM = 2.83 BM
Spin only magnetic moment of the given ion is 2.83 BM. This element contains two unpaired electrons and hence, it is paramagnetic.
APPEARS IN
संबंधित प्रश्न
Choose the most correct option.
Magnetic moment of a metal complex is 5.9 B.M. Number of unpaired electrons in the complex is ______.
Answer the following
Explain trends in ionisation enthalpies of d-block elements.
In 3d series, if nuclear charge increases, the shielding effects will ______.
Transition elements have more tendency to form interstitial compounds because _______.
The catalyst used for decomposition of KClO3 is _______.
Write formula to calculate magnetic moment.
Which alloy is used in the Fischer-Tropsch process in the synthesis of gasoline?
Name the catalyst used in the hydrogenation of ethene to ethane.
Write any four properties of interstitial compounds.
Which transition metal is used as a catalyst in the conversion of inedible oils into solid fat?
Which of the following elements is alloyed with copper to form bronze?
Which of the following elements belongs to first inner transition series?
Which ion has the highest value of theoretical magnetic moment?
The CORRECT order of decreasing ionic radius is:
Which of the following ions will have spin-only magnetic moment equal to 1.73 BM?
Sc3+, Ti3+, Fe2+, Cu2+, Co2+
The spin only magnetic moment of Mn3+ (Z = 25) in aqueous solution is ____________.
How many among the following are ferrous alloys?
Nichrome, chromium steel, bronze, brass, stainless steel, Cupra-nickel.
____________ has the highest electronegativity next to fluorine amongst all the elements.
Which of the following ions has d6 outer electronic configuration?
The following statement is CORRECT EXCEPT:
What is the melting point of zinc?
Write the name of the alloy used in the Fischer Tropsch process in the synthesis of gasoline.
Salts of Ti4+ are colourless. Give reason.
Calculate spin only magnetic moment of divalent cation of transition metal with atomic number 25.
What is the colour of Cu+ ion?
