Advertisements
Advertisements
प्रश्न
Can we use nucleophiles such as NH3, CH3O for the Nucleophilic substitution of alcohols?
उत्तर
1. Increasing order of nucleophilicity,
NH3 < –OH⊕ < CH3O⊖
2. Higher electron density will increase nucleophilicity.
3. Negatively charged species are almost always more nucleophiles than neutral species.
4. RO⊖ has an alkyl group attached, allowing a greater amount of polarizability. This means oxygen’s lone pairs will be more readily available to reach in RO⊖ than in OH⊖. Hence CH3O– is the better nucleophile for the nucleophilic substitution of alcohols. NH3 cannot act as a nucleophile for the nucleophilic substitution of alcohols.
APPEARS IN
संबंधित प्रश्न
Which of the following compounds on reaction with methyl magnesium bromide will give tertiary alcohol.
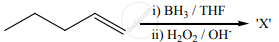
The X is
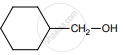 on treatment with Con. H2SO4 predominately gives
on treatment with Con. H2SO4 predominately gives
Suggest a suitable reagent to prepare secondary alcohol with the identical group using a Grignard reagent.
Draw the major product formed when 1-ethoxyprop-1-ene is heated with one equivalent of HI
Predict the major product, when 2-methyl but -2-ene is converted into an alcohol in each of the following method.
Acid catalysed hydration
Predict the major product, when 2-methyl but -2-ene is converted into an alcohol in the following method.
Acid catalysed hydration
Predict the major product, when 2-methyl but -2-ene is converted into an alcohol in the following method.
Acid catalysed hydration
Draw the major product formed when 1-ethoxyprop-1-ene is heated with one equivalent of HI.
Predict the major product, when 2-methyl but -2-ene is converted into an alcohol in each of the following methods.
Acid catalysed hydration
