Advertisements
Advertisements
प्रश्न
Complete flow chart given below.
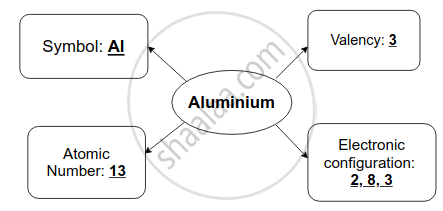
उत्तर
संबंधित प्रश्न
How is copper extracted from its sulphide ore? Explain the various steps supported by chemical equations. Draw labelled diagram for the electrolytic refining of copper.
In the extraction of aluminium: Write the function and formula of cryolite in the extraction of aluminium.
Name two metals which are highly resistant to corrosion.
How is zinc extracted from its carbonate or (calamine)? Explain with equations.
What is meant by the 'concentration of ore'?
What is the difference between a mineral and an ore?
Explain how, mercury is extracted from its sulphide ore (cinnabar). Give equations of the reactions involved.
Give the principles of electromagnetic separation.
State one observation for each of the following :
Copper sulphate solution is electrolysed using copper electrodes.
Name the following:
The process of heating a substance very strongly in such a way that it does not combine with oxygen.
choose the most appropriate term to match the given description.
Heating of the ore in the absence of air to high temperature
What is the difference between calcination and roasting?
Write the name.
The molecular formula of main ore of aluminium –
Write the molecular formulae of the following compound.
Fluorspar
Explain Bayer’s process.
Explain concept with example/explain with the help of a balanced equation.
Calcination
Explain in brief types of extraction of highly reactive metals according to their reactivity.
Which of the following metals exist in their native state in nature?
- Cu
- Au
- Zn
- Ag
2 mL each of concentrated HCl, HNO3 and a mixture of concentrated HCl and concentrated HNO3 in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively. The metal could be
