Advertisements
Advertisements
प्रश्न
Complete the following equations:
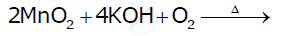
उत्तर

APPEARS IN
संबंधित प्रश्न
Complete the following equation :
`2MnO_4^(-)+6H^++5NO_2^(-)rarr`
Complete the following equation : MnO4- + 8H+ + 5e- →
Complete the following chemical equation
Cr2O72- + 3Sn2+ + 14H+ →
Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
Name a member of the lanthanoid series that is well-known to exhibit +2 oxidation state.
Write the ionic equation showing the oxidation of Fe(II) salt by acidified dichromate solutions.
\[\ce{KMnO4}\] acts as an oxidising agent in alkaline medium. When alkaline \[\ce{KMnO4}\] is treated with \[\ce{KI}\], iodide ion is oxidised to ______.
Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with SO2? Write the ionic equation for the reaction.
