Commerce (English Medium)
Science (English Medium)
Arts (English Medium)
Academic Year: 2015-2016
Date & Time: 9th March 2016, 10:30 am
Duration: 3h
Advertisements
Write the IUPAC name of the given compound:
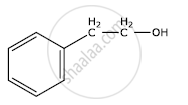
Chapter: [0.05] Coordination Compounds
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
Chapter: [0.06] Haloalkanes and Haloarenes
What is the reason for the stability of colloidal sols?
Chapter: [0.05] Surface Chemistry
Give an example each of a molecular solid and an ionic solid.
Chapter: [0.01] Solid State
Pb(NO3)2 on heating gives a brown gas which undergoes dimerisation on cooling? Identify the gas
Chapter: [0.07] P - Block Elements
For a reaction: 
Rate = k
(i) Write the order and molecularity of this reaction.
(ii) Write the unit of k.
Chapter: [0.03] Chemical Kinetics
Write the chemical equation involved in the following reaction:
Hoffmann-bromamide degradation reaction
Chapter: [0.09] Amines
Write the chemical equations involved in the following reactions:
Carbylamine reaction
Chapter: [0.09] Amines
Gas (A) is more soluble in water than Gas (B) at the same temperature. Which one of the two gases will have the higher value of KH (Henry’s constant) and why
Chapter: [0.01] Solutions
In non-ideal solution, what type of deviation shows the formation of maximum boiling azeotropes?
Chapter: [0.01] Solutions
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex
Chapter: [0.05] Coordination Compounds
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3moles of AgCl are precipitated per mole of the compound. Write (ii) IUPAC name of the complex
Chapter: [0.05] Coordination Compounds
Draw the structures of the following molecules: BrF3
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: XeF4
Chapter: [0.07] P - Block Elements
What happens when: SO2 gas is passed through an aqueous solution Fe+3 salt?
Chapter: [0.07] P - Block Elements
What happens when: XeF4 reacts with SbF5?
Chapter: [0.07] P - Block Elements
Advertisements
Write the final product(s) in each of the following reactions:
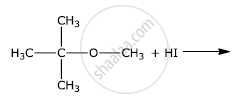
Chapter: [0.06] Haloalkanes and Haloarenes
Write the final product(s) in each of the following reactions:
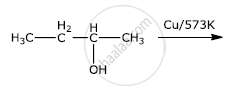
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the final product(s) in each of the following reactions:
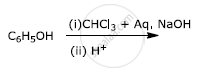
Chapter: [0.07] Alcohols, Phenols and Ethers
How do you convert: Chlorobenzene to biphenyl
Chapter: [0.06] Haloalkanes and Haloarenes
How do you convert: 2-bromobutane to but-2-ene
Chapter: [0.06] Haloalkanes and Haloarenes
Write the major products(s) in the following:
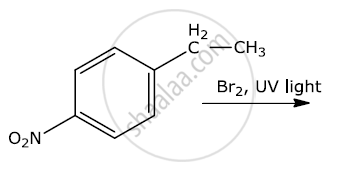
Chapter: [0.09] Amines
Write the major products(s) in the following:
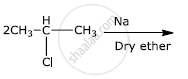
Chapter: [0.06] Haloalkanes and Haloarenes
Write the major products(s) in the following:
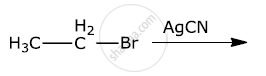
Chapter: [0.06] Haloalkanes and Haloarenes
Write the structural difference between starch and cellulose.
Chapter: [0.1] Biomolecules
What type of linkage is present in nucleic acids?
Chapter: [0.1] Biomolecules
Give one example each for fibrous protein and globular protein.
Chapter: [0.1] Biomolecules
Name the method used for the refining of Nickel metal.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What is the role of cryolite in the extraction of aluminium?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What is the role of limestone in the extraction of iron from its oxides?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Give reasons: SO2 is reducing while TeO2 is an oxidising agent.
Chapter: [0.07] P - Block Elements
Nitrogen does not form pentahalide.
Chapter: [0.07] P - Block Elements
ICl is more reactive than I2.
Chapter: [0.07] P - Block Elements
For the complex [Fe(H2O)6]+3, write the hybridisation, magnetic character and spin of the complex. (At, number : Fe = 26)
Chapter: [0.05] Coordination Compounds
Draw one of the geometrical isomers of the complex [Pt (en)2Cl2] +2 which is optically inactive
Chapter: [0.05] Coordination Compounds
An element crystallises in a b.c.c lattice with cell edge of 500 pm. The density of the element is 7.5g cm-3. How many atoms are present in 300 g of the element?
Chapter: [0.01] Solid State
What is the role of sulphur in the vulcanisation of rubber?
Chapter: [0.15] Polymers
Identify the monomers in the following polymer:
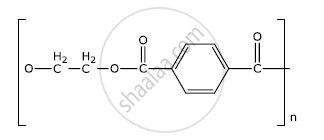
Chapter:
Arrange the following polymers in the Increasing order of their intermolecular forces:
Terylene, Polythene, Neoprene
Chapter:
For the first order thermal decomposition reaction, the following data were obtained:
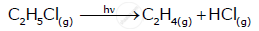
Time / sec Totalpressure / atm
0 0.30
300 0.50
Calculate the rate constant
(Given: log 2 = 0.301, log3 = 0.4771, log 4 = 0.6021)
Chapter: [0.03] Chemical Kinetics
Give reasons for the following:
Aniline does not undergo Friedel- Crafts reaction.
Chapter: [0.09] Amines
Give reasons for the following: (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
Chapter: [0.09] Amines
Give reason for the following:
Primary amines have higher boiling point than tertiary amines.
Chapter: [0.09] Amines
Advertisements
Define the following terms: Lyophilic colloid
Chapter: [0.05] Surface Chemistry
Define the following term:
Zeta potential
Chapter: [0.05] Surface Chemistry
Define the following terms: Associated colloids
Chapter: [0.05] Surface Chemistry
Calculate the boiling point of solution when 4g of MgSO4 (M= 120 g mol-1) was dissolved in 100g of water, assuming MgSO4 undergoes complete ionization. (Kb for water = 0.52 K kgmol-1)
Chapter: [0.01] Solutions
Due to hectic and busy schedule, Mr Angad made his life full of tensions and anxiety. He started taking sleeping pills to overcome the depression without consulting the doctor. Mr Deepak, a close friend of Mr. Angad advised him to stop taking sleeping pills and suggested to change his life lifestyle by doing yoga, meditation and some physical exercise. Mr. Angad followed his friend’s advice and after few days he started feeling better.
After reading the above passage, answer the following
(i) What are the values (at least two) displayed by Mr. Deepak?
(ii) Why is it not advisable to take sleeping pills without consulting doctor?
(iii) What are tranquilisers? Give two examples.
Chapter: [0.16] Chemistry in Everyday Life
Write the structures of A and B in the following reactions:

Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the structures of A and B in the following reactions
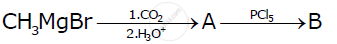
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Distinguish between:
C6H5-COCH3 and C6H5-CHO
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Distinguish between: CH3COOH and HCOOH
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Arrange the following in the increasing order of their boiling points: CH3CHO, CH3COOH, CH3CH2OH
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the chemical reaction involved in Wolff-Kishner reduction.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Arrange the following in the increasing order of their reactivity towards nucleophilic addition reaction:
C6H5COCH3, CH3-CHO, CH3COCH3
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Why carboxylic acid does not give reactions of carbonyl group?
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the product in the following reaction
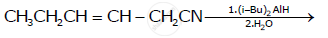
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
A and B are two functional isomers of compound C3H6O.On heating with NaOH and I2, isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Calculate E°cell for the following reaction at 298 K:
2Al(s) + 3Cu+2(0.01M) → 2Al+3(0.01M) + 3Cu(s)
Given: Ecell = 1.98V
Chapter: [0.02] Electrochemistry
Using the E° values of A and B, predict which is better for coating the surface of iron [E°(Fe+2/Fe) = -0.44V] to prevent corrosion and why?
Given: E° (A+2/A)=-2.37 V: E°(B+2/B)= -0.14V
Chapter: [0.02] Electrochemistry
The conductivity of 0.001 mol L-1 solution of CH3COOH is 3.905× 10-5 S cm-1. Calculate its molar conductivity and degree of dissociation (α) Given λ°(H+)= 349.6 S cm2 mol-1 and λ°(CH3COO)= 40.9S cm2mol-1.
Chapter: [0.02] Electrochemistry
Define electrochemical cell
Chapter: [0.02] Electrochemistry
What happens if external potential applied becomes greater than E°cell of electrochemical cell?
Chapter: [0.02] Electrochemistry
Account for the following:
Mn shows the highest oxidation state of +7 with oxygen but with fluorine, it shows oxidation state of +4.
Chapter: [0.04] d-block and f-block Elements
Account for the following:
Cr2+ is a strong reducing agent.
Chapter: [0.04] d-block and f-block Elements
Account for the following:
Cu+2 salts are coloured, while Zn2+ salts are white.
Chapter: [0.04] d-block and f-block Elements
Complete the following equations:
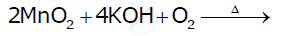
Chapter: [0.04] d-block and f-block Elements
Complete the following equations: Cr2O72- + 14H+ + 6I →
Chapter: [0.04] d-block and f-block Elements
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Write the element which shows maximum number of oxidation states. Give reason.
Chapter: [0.04] d-block and f-block Elements
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Which element has the highest m.p?
Chapter: [0.04] d-block and f-block Elements
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Which element shows only +3 oxidation state?
Chapter: [0.04] d-block and f-block Elements
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Which element is a strong oxidising agent in +3 oxidation state and why?
Chapter: [0.04] d-block and f-block Elements
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2015 - 2016
Previous year Question paper for CBSE Class 12 -2016 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
