Advertisements
Advertisements
प्रश्न
What is the role of sulphur in the vulcanisation of rubber?
उत्तर १
Rubber is made of hydrocarbon chains, basically carbon and hydrogen. These chains slide over one another and get tied to one another which leads to natural rubber being sticky. When sulphur is used in the vulcanisation process, it reacts with these chains and forms disulphide (or similar) bonds. Sulphur forms cross linkages at the reactive sites of the double bonds because of which rubber gets stiffened.
उत्तर २
In vulcanization, sulphur forms cross-link at the reactive sites of double bonds of natural rubber so the rubber gets stiffened.
संबंधित प्रश्न
What is natural rubber?
(A) Cis-1,4-polyisoprene
(B) Neoprene
(C) Trans-1,4-polyisoprene
(D) Butyl rubber
Write names and chemical formulae of monomers used in preparing Buna-S.
Discuss the main purpose of vulcanisation of rubber.
Identify the monomer in the following polymeric structures.
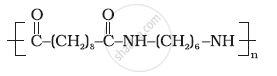
Write names and chemical formulae of monomers used in preparing Buna-N.
What is the role of Sulphur in the vulcanization of rubber?
Vulcanisation makes rubber:
(i) more elastic
(ii) soluble in inorganic solvent
(iii) crystalline
(iv) more stiff
Which of the following statement is CORRECT regarding the drawbacks of raw rubber?
In vulcanization of rubber:
Match List I with List II.
| List I | List II |
| (Monomer Unit) | (Polymer) |
| (a) Caprolactum | (i) Natural rubber |
| (b) 2-Chloro-1, 3-butadiene | (ii) Buna-N |
| (c) Isoprene | (iii) Nylon-6 |
| (d) Acrylonitrile | (iv) Neoprene |
Choose the correct answer from the options given below:
