Advertisements
Advertisements
प्रश्न
Consider the following salt:
NH4X
If in salt NH4X, X is nitrate, then its solution will give what colour with a universal indicator? Why?
उत्तर
If X is a nitrate in salt NH4X, its solution will be green with a universal indicator. This is because ammonium ion \[\ce{(NH^+4)}\] is a weak acid that can partially dissociate in the solution to form hydrogen ions (H+), making the solution acidic. The ion nitrate \[\ce{(NO^-3)}\] is a neutral ion that has no effect on pH. The solution's green colour indicates a pH between 7 and 6.
संबंधित प्रश्न
When phenolphthalein is added to NaOH, the colour of the solution will become _________.
- colourless
- red
- pink
- yellow
Hydrochloric acid reacts with a metal X to form a gas Y which burns with a 'pop' sound. Sodium hydroxide solution also reacts with the same metal X (on heating) to form the same gas Y.
Write the chemical equation of the reaction of metal X with (i) hydrochloric acid, and (ii) sodium hydroxide solution.
Write the chemical formula of soda ash?
Answer the following question:
What is the universal indicator? Does Mg (OH)2 react with sodium hydroxide? If not, why?
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
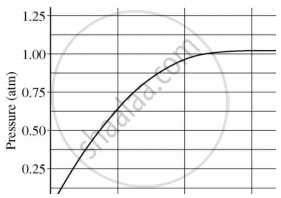
Which of the following salts does not contain any water of crystallisation?
Which of the following is used for dissolution of gold?
Solutions of acids conduct ______.
Can we taste acids and bases to identify them?
Consider the following salt:
\[\ce{ZCO3}\]
What would be the change in colour in blue litmus if \[\ce{ZCO3}\] is added to it and Z is potassium?
