Advertisements
Advertisements
प्रश्न
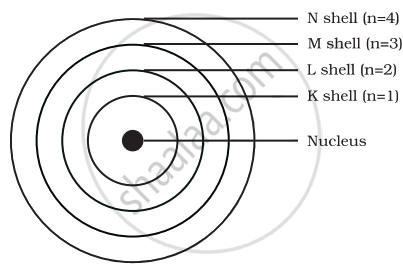
Draw a sketch of Bohr’s model of an atom with three shells.
उत्तर
According to Bohr's atomic model with three orbitals, the order of the three orbitals will be K, L and M
The number of electrons in each orbit = 2n2
Number of electrons in K orbit = 2 × 12 = 2
Number of electrons in L orbit = 2 × 22 = 8
Number of electrons in M orbit = 2 × 32 = 18
But the maximum number of electrons in the outer orbit can be 8.
APPEARS IN
संबंधित प्रश्न
Describe Bohr’s model of the atom.
Name the central part of an atom where protons and neutrons are held together.
What are the various letters used by Bohr to represent electron shells in an atom?
State true or false. If false, correct the statement.
Smaller the size of the orbit, lower is the energy of the orbit.
Explain the postulates of Bohr’s atomic model.
An atom with 3 protons and 4 neutrons will have a valency of
Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?
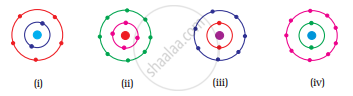
What are the Limitations of Bohr’s Model?
The atomic model based on quantum theory was first proposed by ______.
The scientist who proposed the atomic model based on the quantisation of energy for the first time is ______.
