Advertisements
Advertisements
प्रश्न
Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?
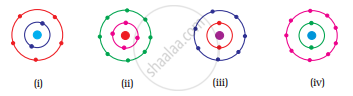
विकल्प
(i) and (ii)
(ii) and (iii)
(ii) and (iv)
(i) and (iv)
उत्तर
(ii) and (iv)
Explanation -
Fig. (ii) contains 4 electrons in K shell and fig. (iv) contains 9 electrons in L shell which are not in accordance with Bohr’s model.
APPEARS IN
संबंधित प्रश्न
Draw a sketch of Bohr’s model of an atom with three shells.
Describe Bohr’s model of the atom.
Name the scientists who described the arrangement of electrons in an atom.
What are the various letters used by Bohr to represent electron shells in an atom?
In the figure given alongside
(a) Name the shells denoted by A,B, and C. Which shell has least energy
(b) Name X and state the charge on it
(c) The above sketch is of …………. Model of an atom
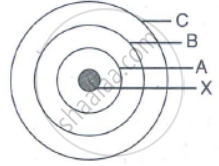
State true or false. If false, correct the statement.
Smaller the size of the orbit, lower is the energy of the orbit.
An atom with 3 protons and 4 neutrons will have a valency of
Niels Bohr was Born on ______.
The atomic model based on quantum theory was first proposed by ______.
Bohr's model of atoms ______.
