Advertisements
Advertisements
प्रश्न
Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?
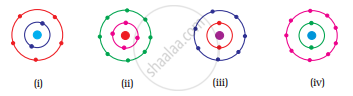
पर्याय
(i) and (ii)
(ii) and (iii)
(ii) and (iv)
(i) and (iv)
उत्तर
(ii) and (iv)
Explanation -
Fig. (ii) contains 4 electrons in K shell and fig. (iv) contains 9 electrons in L shell which are not in accordance with Bohr’s model.
APPEARS IN
संबंधित प्रश्न
Draw a sketch of Bohr’s model of an atom with three shells.
Compare all the proposed models of an atom given in this chapter.
Name the scientists who described the arrangement of electrons in an atom.
Name the central part of an atom where protons and neutrons are held together.
Describe Bohr's model of the atom. How did Neils Bohr explain the stability of atom?
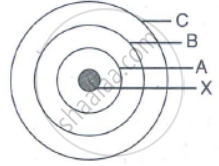
In the figure given alongside
(a) Name the shells denoted by A,B, and C. Which shell has least energy
(b) Name X and state the charge on it
(c) The above sketch is of …………. Model of an atom
Explain the postulates of Bohr’s atomic model.
What are the Limitations of Bohr’s Model?
The atomic model based on quantum theory was first proposed by ______.
The scientist who proposed the atomic model based on the quantisation of energy for the first time is ______.
