Advertisements
Online Mock Tests
Chapters
2: Is Matter Around Us Pure
3: Atoms and Molecules
▶ 4: Structure of the Atom
5: The Fundamental Unit of Life
6: Tissues
7: Diversity In Living Organisms
8: Motion
9: Force and Laws of Motion
10: Gravitation
11: Work and Energy
12: Sound
13: Why Do We Fall Ill
14: Natural Resources
15: Improvement In Food Resources
![NCERT Exemplar solutions for Science [English] Class 9 chapter 4 - Structure of the Atom NCERT Exemplar solutions for Science [English] Class 9 chapter 4 - Structure of the Atom - Shaalaa.com](/images/science-english-class-9_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 4: Structure of the Atom
Below listed, you can find solutions for Chapter 4 of CBSE NCERT Exemplar for Science [English] Class 9.
NCERT Exemplar solutions for Science [English] Class 9 4 Structure of the Atom Multiple Choice Questions [Pages 26 - 30]
Which of the following correctly represent the electronic distribution in the Mg atom?
3, 8, 1
2, 8, 2
1, 8, 3
8, 2, 2
Rutherford’s ‘alpha (α) particles scattering experiment’ resulted in to discovery of
Electron
Proton
Nucleus in the atom
Atomic mass
The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
`""_15^13"X"`
`""_31^16"X"`
`""_16^15"X"`
`""_15^16"X"`
Dalton’s atomic theory successfully explained
(i) Law of conservation of mass
(ii) Law of constant composition
(iii) Law of radioactivity
(iv) Law of multiple proportions
(i), (ii) and (iii)
(i), (iii) and (iv)
(ii), (iii) and (iv)
(i), (ii) and (iv)
Which of the following statements about Rutherford’s model of atom are correct?
(i) considered the nucleus as positively charged
(ii) established that the α–particles are four times as heavy as a hydrogen atom
(iii) can be compared to solar system
(iv) was in agreement with Thomson’s model
(i) and (iii)
(ii) and (iii)
(i) and (iv)
only (i)
Which of the following are true for an element?
- Atomic number = number of protons + number of electrons
- Mass number = number of protons + number of neutrons
- Atomic mass = number of protons = number of neutrons
- Atomic number = number of protons = number of electrons
(i) and (ii)
(i) and (iii)
(ii) and (iii)
(ii) and (iv)
In the Thomson’s model of atom, which of the following statments are correct?
- the mass of the atom is assumed to be uniformaly distributed over the atom
- the positive charge is assumed to be uniformaly distributed over the atom
- the electrons are uniformaly distributed in the positively charged sphere
- the electrons attract each other to stabilise the atom
(i), (ii) and (iii)
(i) and (iii)
(i) and (iv)
(i), (iii) and (iv)
Rutherford’s α–particle scattering experiment showed that
- electrons have negative charge
- the mass and positive charge of the atom is concentrated in the nucleus
- neutron exists in the nucleus
- most of the space in atom is empty Which of the above statements are correct?
(i) and (iii)
(ii) and (iv)
(i) and (iv)
(iii) and (iv)
The ion of an element has 3 positive charges. The mass number of atom of this element is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
13
10
14
16
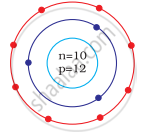
Identify the Mg2+ ion from the Fig. where, n and p represent the number of neutrons and protons respectively
In a sample of ethyl ethanoate (CH3COOC2H5), the two oxygen atoms have the same number of electrons but different number of neutrons. Which of the following is the correct reason for it ?
one of the oxygen atoms has gained electrons
one of the oxygen atoms has gained protons
the two oxygen atoms are isotopes
the two oxygen atoms are isobars
Elements with valency 1 are
always metals
always metalloids
either metals or non-metals
always non-metals
The first model of an atom was given by :
Neils Bohr
Ernest Rutherford
J.J. Thomson
Eugen Goldstein
An atom with 3 protons and 4 neutrons will have a valency of
3
7
1
4
The electron distribution in an aluminium atom is
2, 8, 3
2, 8, 2
8, 2, 3
2, 3, 8
Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?
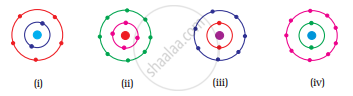
(i) and (ii)
(ii) and (iii)
(ii) and (iv)
(i) and (iv)
Which of the following statement is always correct?
An atom has equal number of electrons and protons.
An atom has equal number of electrons and neutrons.
An atom has equal number of protons and neutrons.
An atom has equal number of electrons, protons and neutrons.
Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order
- Rutherford’s atomic model
- Thomson’s atomic model
- Bohr’s atomic model
(i), (ii) and (iii)
(ii), (iii) and (i)
(ii), (i) and (iii)
(iii), (ii) and (i)
NCERT Exemplar solutions for Science [English] Class 9 4 Structure of the Atom Short Answer Questions [Pages 30 - 32]
Is it possible for the atom of an element to have one electron, one proton and no neutron. If so, name the element.
Write any two observations which support the fact that atoms are divisible.
Will 35Cl and 37Cl have different valencies? Justify your answer.
Why did Rutherford select a gold foil in his α–ray scattering experiment?
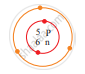
Find out the valency of the atoms represented by the Fig (a) and (b).

One electron is present in the outermost shell of the atom of an element X. What would be the nature and value of charge on the ion formed if this electron is removed from the outermost shell?
Write down the electron distribution of chlorine atom. How many electrons are there in the L shell? (Atomic number of chlorine is 17).
In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed?
What information do you get from the Fig. about the atomic number, mass number and valency of atoms X, Y and Z? Give your answer in a tabular form.
 |
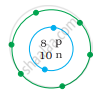 |
 |
| (X) | (Y) | (Z) |
In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons. Do you agree with the statement? Justify your answer.
Calculate the number of neutrons present in the nucleus of an element X which is represented as `""_15^31"X"`
Match the names of the Scientists given in column A with their contributions towards the understanding of the atomic structure as given in column B
| (A) | (B) | ||
| (a) | Ernest Rutherford | (i) | Indivisibility of atoms |
| (b) | J.J.Thomson | (ii) | Stationary orbits |
| (c) | Dalton | (iii) | Concept of nucleus |
| (d) | Neils Bohr | (iv) | Discovery of electrons |
| (e) | James Chadwick | (v) | Atomic number |
| (f) | E. Goldstein | (vi) | Neutron |
| (g) | Mosley | (vii) | Canal rays |
The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
Complete the Table on the basis of information available in the symbols given below
- `""_17^35"Cl"`
- `""_6^12"C"`
- `""_35^81"Br"`
| Element | np | nn |
Helium atom has 2 electrons in its valence shell but its valency is not 2, Explain
Fill in the blanks in the following statements
Rutherford’s α-particle scattering experiment led to the discovery of the ———
Isotopes have same ———but different———.
Neon and chlorine have atomic numbers 10 and 17 respectively. Their valencies will be ——— and ———respectively.
The electronic configuration of silicon is ——— and that of sulphur is ———.
An element X has a mass number 4 and atomic number 2. Write the valency of this element?
NCERT Exemplar solutions for Science [English] Class 9 4 Structure of the Atom Long Answer Questions [Page 32]
Why do Helium, Neon and Argon have a zero valency?
The ratio of the radii of hydrogen atom and its nucleus is ~ 105. Assuming the atom and the nucleus to be spherical,
- what will be the ratio of their sizes?
- If atom is represented by planet earth ‘Re ’ = 6.4 ×106 m, estimate the size of the nucleus.
Enlist the conclusions drawn by Rutherford from his α-ray scattering experiment.
In what way is the Rutherford’s atomic model different from that of Thomson’s atomic model?
What were the drawbacks of Rutherford’s model of an atom?
What are the postulates of Bohr’s model of an atom?
Show diagrammatically the electron distributions in a sodium atom and a sodium ion and also give their atomic number.
In the Gold foil experiment of Geiger and Marsden, that paved the way for Rutherford’s model of an atom, ~ 1.00% of the α-particles were found to deflect at angles > 50º. If one mole of α-particles were bombarded on the gold foil, compute the number of α-particles that would deflect at angles less than 500.
Solutions for 4: Structure of the Atom
![NCERT Exemplar solutions for Science [English] Class 9 chapter 4 - Structure of the Atom NCERT Exemplar solutions for Science [English] Class 9 chapter 4 - Structure of the Atom - Shaalaa.com](/images/science-english-class-9_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
NCERT Exemplar solutions for Science [English] Class 9 chapter 4 - Structure of the Atom
Shaalaa.com has the CBSE Mathematics Science [English] Class 9 CBSE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT Exemplar solutions for Mathematics Science [English] Class 9 CBSE 4 (Structure of the Atom) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. NCERT Exemplar textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Science [English] Class 9 chapter 4 Structure of the Atom are Existence of Charged Particles in Matter, Atoms: Building Blocks of Matter, Discovery of Charged Particles in Matter, Protons (p), Electrons (e), Neutrons (n), J. J. Thomson’s Atomic Model, Lord Rutherford’s Atomic model, Neils Bohr’s Model of an Atom, Electronic Configuration of Atom, Atomic Number (Z), Mass Number (A), and Number of Neutrons (n), Isotopes, Isobars, Atoms and Molecules Numericals, Valency, Uses of Radioactive Isotopes, Advantage and Limitations of Thomson’s Atomic Model, Limitations of Rutherford’s Atomic Model, Different Ways to Determine Valency, Atomic Mass.
Using NCERT Exemplar Science [English] Class 9 solutions Structure of the Atom exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in NCERT Exemplar Solutions are essential questions that can be asked in the final exam. Maximum CBSE Science [English] Class 9 students prefer NCERT Exemplar Textbook Solutions to score more in exams.
Get the free view of Chapter 4, Structure of the Atom Science [English] Class 9 additional questions for Mathematics Science [English] Class 9 CBSE, and you can use Shaalaa.com to keep it handy for your exam preparation.