Advertisements
Advertisements
प्रश्न
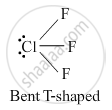
Draw the structures of the following : ClF3
उत्तर
Structure of ClF3
APPEARS IN
संबंधित प्रश्न
Draw the structures of the following molecules: BrF3
Draw the structure of BrF5
Explain why:
(1) Interhalogen compounds are more reactive than the related elemental halogens.
(2) Sulphur exhibits the tendency for catenation but oxygen does not.
(3) On being slowly passed through water, PH3 forms bubbles but NH3 dissolves.
Find the INCORRECT match.
Which among the following is the most reactive?
Which of the following interhalogen is a colourless liquid at room temperature?
The number of lone pair of electrons present on central atom in the interhalogen XX'3 molecule:
Which among the following halogen does not form polyhalide ion?
Which of the following is not the characteristic of interhalogen compounds?
In the interhalogen compound AB3, the state of hydridisation of A is ______.
