Advertisements
Advertisements
Question
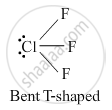
Draw the structures of the following : ClF3
Solution
Structure of ClF3
APPEARS IN
RELATED QUESTIONS
Draw structure of Chlorine trifluoride
ICl is more reactive than I2.
Explain why:
(1) Interhalogen compounds are more reactive than the related elemental halogens.
(2) Sulphur exhibits the tendency for catenation but oxygen does not.
(3) On being slowly passed through water, PH3 forms bubbles but NH3 dissolves.
Which among the following pairs of halogen forms the interhalogen compound of the type \[\ce{XX^{'}_7}\]?
What is the oxidation state of bromine in the product?
\[\ce{Br2 + \underset{(excess)}{3F2} ->?}\]
Which among the following is the most reactive?
What type of hybridization is observed in interhalogen compounds of the type \[\ce{XX^'_3}\]?
Match the compounds given in Column I with the hybridisation and shape given in Column II and mark the correct option.
| Column I | Column II |
| (A) XeF6 | (1) sp3d3 – distorted octahedral |
| (B) XeO3 | (2) sp3d2 – square planar |
| (C) XeOF4 | (3) sp3 – pyramidal |
| (D) XeF4 | (4) sp3 d2 – square pyramidal |
In the interhalogen compound AB3, the state of hydridisation of A is ______.
Brown ring test is used for detection of which radical?
