Advertisements
Advertisements
Questions
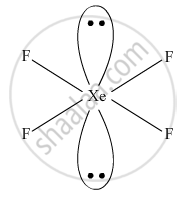
Draw the structures of the following molecules: XeF4
Write the structures of the following:
XeF4
Solution
XeF4, Square planar
APPEARS IN
RELATED QUESTIONS
Balance the following equation: XeF6 + H2O → XeO2F2 + HF
How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Write electronic configuration and two uses of neon. (Z = 10)
Complete the following reactions:
XeF6+2H2O ----->
Draw structure and name the shape of bromine trifluoride.
Which of the following fluorides does not exist?
In which of the following pairs, the two species are isostructural:
Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
\[\ce{XeF6 + H2O ->[Partial][Hydrolysis] \underline{}\underline{}\underline{}\underline{} + \underline{}\underline{}\underline{}\underline{}}\]
Discuss the trends in electronegativity and atomic radii for elements of group 16 17, 18.
